Alternative management of intractable chylous ascites following robot-assisted pancreaticoduodenectomy of Viscum album sclerotherapy: a case report
Article information
Abstract
A patient showed signs of fever and Hemovac insertion site discharge 8 days after surgery and was admitted. Abdominal paracentesis found milky ascites with triglyceride levels of the peritoneal fluid as high as 1,603 g/mL. Diagnosed as chylous ascites, symptomatic therapy such as empirical antibodies and diuretics was administered with paracentesis before being discharged. The ascites volume increased again, and the patient was re-admitted. The patient was treated with orlistat, octreotide, total parenteral nutrition administration, ascites drainage, and diuretics. Ascites levels increased further and intraperitoneal Viscum was administered. Clear ascites was observed, and the patient was discharged. We reported a case where conventional treatment for chylous ascites that occurred after pancreaticoduodenectomy was shown to be ineffective while Viscum extracted from mistletoe was able to manage chylous ascites.
INTRODUCTION
Chylous ascites (CA) is a rare disease that is defined as the extravasation of triglyceride (TG)-rich lymph into the peritoneal cavity [1]. Its occurrence is related to injury and rupture of the lymphatics or increased peritoneal lymphatic pressure due to obstruction. Malignant tumors and cirrhosis are known to account for approximately two-thirds of all cases of CA in Western countries, and it is also known to be related to trauma, infection, surgery, cardiac and congenital diseases. Particularly, postoperative CA is becoming a more important clinical issue in patients after major abdominal surgery because of the increase in extended resection and lymph node dissection.
Since CA elicit symptoms such as abdominal distention, pain, weight gain/loss, dyspnea, malnutrition, edema, nausea, enlarged lymph node, and early satiety, it is very important to diagnose CA and treat it as early as possible [2]. Definitive diagnosis of CA is established by measuring the TG level. If the concentration is measured above 200 mg/dL, the diagnosis of CA is confirmed [3]. Conservative treatments of CA include total parenteral nutrition (TPN) or high-protein, low-fat diet with medium chain triglyceride (MCT). In addition, somatostatin, octreotide, and agents such as orlistat have been used to successfully treat the disease. Surgery may be an option for CA refractory to conservative therapy. According to case reports describing operative procedures in CA, surgical interventions consist of transjugular intrahepatic portosystemic shunt, peritoneovenous shunt, angiography with embolization of leaking vessel, and laparotomy. However, surgery could be very challenging in many postoperative CA cases. It is difficult to locate the site of the chyle leakage, and the patient may have poor general condition and adhesion because of previous surgery. In addition, conservative care is known to be effective in almost all cases of CA which makes surgical approach an unfavorable option.
Viscum album, commonly known as European mistletoe, is a medical plant widely used for anticancer adjuvant treatment in Europe and Republic of Korea for its function of stimulating the immune system. V. album extract also acts as a sclerosant and it was proven to be effective in treating malignant pleural effusion in many studies [4]. It was also shown to be successful in the treatment of secondary spontaneous pneumothorax in elderly patients [5].
Herein, we reported a case of postoperative CA occurred after robot-assisted pylorus preserving pancreaticoduodenectomy, which was successfully treated with consecutive Viscum sclerotherapy. The study’s protocol was reviewed and approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2022-0612). Informed consent was waived because the study had a retrospective nature and the analysis used anonymous clinical data.
CASE REPORT
Case presentation
In September 2021, a 56-year-old female patient was admitted for further evaluation of a suspected resectable pancreas head cancer. It was suspected during the evaluation of jaundice in the primary hospital. She had a history of diabetes mellitus, chronic B viral hepatitis, and subclinical hypothyroidism. She had no history of smoking, nor drinking excessively. There were no abnormal findings in review of systems and physical examination.
Out-side images of abdomen-pelvic computerized tomography and magnetic resonance imaging showed a 2 cm probably resectable periampullary mass and pancreatic mass. Endoscopic retrograde cholangiopancreatography showed ulcerative change with spontaneous oozing at ampulla of Vater (AoV), suggestive of AoV cancer. Biopsy was done at AoV. Positron emission tomography-computed tomography (Fig. 1A) demonstrated a mass of intense F-18 fluorodeoxyglucose (FDG) uptake in the periampullary area and no significant FDG uptake in lymph nodes, suggestive of malignancy in the periampullary area with no lymph node metastasis. Pathology results revealed well differentiated adenocarcinoma in both pancreas head and AoV. It was concluded that the patient’s most probable diagnosis is resectable AoV cancer with no lymph node or distant metastasis. Upfront surgery was planned, and adjuvant therapies were planned to be decided after the surgery.
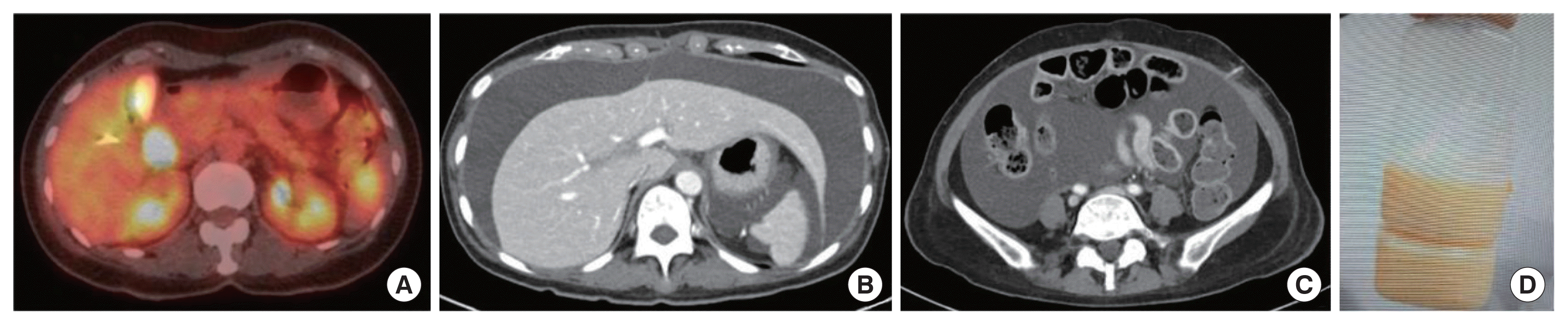
Preoperative imaging work up and image of chylous ascites after surgery. (A) Positron emission tomography-computed tomography (PET-CT) demonstrated a mass of intense F-18 fluorodeoxyglucose (FDG) uptake in the periampullary area and no lymph node with significantly increased FDG uptake in the abdomen. (B, C) CT showed markedly increased amount of ascites at hospital day 11 of 3rd admission (POD62). (D) On hospital day 1 of 3rd admission (POD52), 2,400 mL drained milky ascites. POD, postoperative day.
Operative finding
The patient underwent robot-assisted pylorus preserving pancreaticoduodenectomy on October 18, 2021. Pancreaticojejunostomy was performed duct-to-mucosa with no stent, hepatojejunostomy was performed with posterior continuous and anterior interrupted absorbable sutures, and duodenojejunostomy was done side to side with continuous absorbable sutures. There was moderate adhesion around hepatoduodenal ligament, uncinate process, and superior mesenteric artery (SMA). SMA lateral border was dissected without neurectomy. Arteries directly going into the pancreas were all individually controlled by an energy device. Gastroduodenal artery was ligated using Endo GIA. Right gastric artery and vein were all clipped and divided. Additional frozen samplings were not done since bile duct resection and pancreatic resection margin seemed adequate.
Pathologic examination
Pathological diagnosis of the specimen was AoV cancer: adenocarcinoma, moderately differentiated. Gross finding for the specimen included an infiltrative tumor directly invading pancreas up to 0.5 cm (pT3a), which measured 2.2×1.8 cm. Lymphovascular invasion and perineural invasion were present. All resection margins including the common bile duct, pancreatic parenchymal and duct margin, SMA margin, superior mesenteric vein/portal vein groove, anterior and posterior surface, proximal duodenum, and distal duodenum were negative for carcinoma cells. Gallbladder was also free of carcinoma. A total of 17 lymph nodes were retrieved without any positive nodes (pN0). Mesenteric soft tissue was negative for carcinoma cells.
Initial postoperative course
In general, the patient showed uneventful postoperative recovery. Postoperative pancreatic fistula was noted on a postoperative day (POD)3, however, it spontaneously disappeared. The patient’s general status remained tolerable, and she was discharged on POD11.
Readmission due to CA
The patient visited the emergency department with ascites-like oozing and fever at the percutaneous transhepatic biliary drainage site and complained of abdominal distention on POD19. Abdomen-pelvic computed tomography confirmed markedly increased ascites rule out bacterial peritonitis, and the patient was admitted for ascites drainage and conservative care (Fig. 1B and C). Ascites analysis performed after ascites drainage showed that the serum-ascites albumin ratio is 1.3 g/dL, ascites albumin is 2.2 g/dL, white blood cells is 2,307/μL, and polymorphonuclear leukocyte is 375/μL. Bacterial infection was not confirmed in both culture and gram stains performed in peritoneal fluid and blood. At the time of admission, a mixed bloody color was seen, but a milky straw color was observed in subsequent examinations (Fig. 1D). On POD20, the TG of the peritoneal fluid was confirmed to be 1,603 mg/dL, and the patient was treated under the diagnosis of CA due to lymphatic vessel injury during operation.
CA management
Ascites drainage and albumin replacement is performed for patients complaining of abdominal distension due to CA using furosemide and spironolactone for ascites control, orlistat for lipid lowering, octreotide for portal pressure and lipid lowering, and Viscum 0.02 mg injection for immunotherapy. Lymphangiogram and embolization were not performed because the success rate was considered to be low according to interventional radiology. The body weight and abdominal circumference are measured for follow-up.
Due to the discomfort caused by the continuing ascites, high-calorie TPN was administered from POD77. Ascites improved for a while in the beginning, but it worsened again so the patient was returned to the general diet on POD89. Because milky and high TG ascites continued to drain, intraabdominal Viscum shooting was performed for the treatment of lymphatic sclerosing. The dosage and administration interval of V. album were determined according to the intraperitoneal application protocol of AbnobaViscum and were adjusted according to the characteristics of the patient’s ascites and the amount of drainage. The ascites recovered to a serous color after a 5A (1A=20 mg) Viscum injection on POD94 and a 10A Viscum injection on POD97. Subsequently, during the fourth hospitalization, Viscum 5A and 10A were injected on POD110 and POD115. However, the ascites condition did not sufficiently respond to the treatment. The dose and administration interval were adjusted from the currently used protocol in consideration of the patient’s condition. Viscum 15A, 20A, and 20A were injected on POD126, POD129, and POD132, respectively, and ascites improved (Fig. 2A). There was mild fever around 38°C and C-reactive protein elevation after Viscum shooting, but it was normalized by conservative management including antibiotics. After Viscum treatment, ascites drainage decreased and the color changed to serous. In addition, body weight and abdominal circumference had a decreasing trend along with dissolved ascites in abdominal computed tomography (Fig. 2B and C). The patient was in good general condition at POD156, and the follow-up after 3 months also showed no sign of relapse.

Treatment and course of chylous ascites. (A) Body weight and abdominal circumference change with drainage, total parenteral nutrition, and Bactrim injection. The double slash on the graph represents the unrecorded non-hospitalization period. The blue and red bars indicate drainage fluid, chylous and serous, respectively, and the amount of drainage is described below the bar. The orange arrow means shooting of Viscum with normal saline. (B, C) Abdominal computed tomography with contrast taken on 5 months after surgery during outpatient follow-up. It showed mostly cleared ascites with catheterization.
DISCUSSION
Postoperative CA, characterized by a collection of chyle, or milky-appearing fluid in the peritoneal cavity that is rich in TGs, is assumed to be caused by the direct injury of the main lymphatic ducts, its branches, or lymph nodes during the operation. After surgery, CA can occur within a week due to direct injury of the lymphatic channel or it can occur after a couple of weeks or even months due to the obstruction of lymphatic channels by surrounding tissue. Postoperative CA causes prolonged hospital stays and considerable increase of healthcare costs, along with morbidity such as malnutrition, dehydration, immunosuppression, or septic complications, and therefore it is important to provide early and appropriate treatment when diagnosed. The incidence of postoperative CA varies between different types of surgery and the extent of lymph node dissection, with the highest incidence after pancreatic surgery which is up to 11.0% [6].
Postoperative CA is mostly managed with conservative treatment. Conservative measures focus on the maintenance of optimal nutritional balance and reduction of the amount of lymph formation and flow. The initial step of CA management is dietary adjustment to a low-fat, high-protein diet with MCTs, which successfully resolves CA in up to 50% of cases. When there is no improvement, second line therapies including TPN, somatostatin, or its analogue, octreotide must be administered. Resolution rates were reported from 60% to 100% in these therapies [7–9].
In this case, various conservative treatments and procedure such as drainage and albumin replacement, diuretics, orlistat, octreotide, and TPN were tried to treat CA following pancreaticoduodenectomy. However, the amount of ascites continued to increase, and none of the treatments were effective. There are a number of cases reported to be refractory to conservative management and additional therapeutic strategies were trialed in these cases, including surgical management. However, surgical management in this case was predicted to have high morbidity since the site of the leakage is not easy to find even with the help of lymphoscintigraphy or lymphangiogram, and the patient may not be able to tolerate an additional surgery after a major abdominal surgery.
V. album, a European mistletoe, can act as sclerosant and it was previously proven effective to treat malignant pleural effusion and malignant ascites in many studies [10]. Viscum was also successfully used in chemical pleurodesis for secondary spontaneous pneumothorax in elderly patients [5]. V. album is known to be a considerably safe agent with mild adverse effects compared to other sclerotic agents, such as Picibanil (OK-432) which was used as a sclerosing agent in lymphatic malformation and in some cases of lymphoceles or intractable chylous leak after neck dissection [11–13]. Based on this understanding, the surgical team attempted to treat the patient by V. album shooting through the drain for lymphatic sclerosing. After administration, fever and inflammation markers mildly increased, but they were controlled with conservative treatment, and the amount of ascites drainage gradually decreased, and the characteristic changed to serous. Also, body weight and abdominal circumference also showed a tendency to decrease, confirming that CA was treated.
To the best of our knowledge, this is the first reported case of successful CA treatment using V. album injection. Although further research is required to determine the indication and the clinical efficacy of V. album sclerotherapy, this treatment is expected to be a novel minimal invasive treatment alternative for patients with CA who are unresponsive or expected to have a low success rate with conventional treatments.
Notes
This study was partly supported by Abnoba Korea research grant (4-2021-0077): oncologic impact of postoperative AbnobaViscum injection in resected pancreatic cancer. Except for that, no potential conflict of interest relevant to this article was reported.
FUNDING
This study was partly supported by Abnoba Korea research grant (4-2021-0077): oncologic impact of postoperative AbnobaViscum injection in resected pancreatic cancer.
