Is a cutoff value of 12 still useful in stage II right-sided colon cancer without risk factors?
Article information
Abstract
Purpose
Various clinical practice guidelines recommend at least 12 regional lymph nodes should be removed for resected colon cancer. According to a recent study, the lymph node yield (LNY) in colon cancer surgery in the last 20 years has tended to increase from 14.91 to 21.30. However, it is unclear whether these guidelines adequately reflect recent findings on the number of harvested lymph nodes in colon cancer surgery. The aim of this study is to assess the impact of an LNY of more than 25 on survival in right-sided colon cancer.
Methods
We included 285 patients who underwent a right hemicolectomy during the period from January 2010 through December 2015. Patients were divided into two groups (<25 nodes and ≥25 nodes). Primary endpoints included 5-year and 10-year survival including disease-free and overall.
Results
We found that survival outcomes of patients with a harvest of ≥25 nodes were not significantly different compared with a <25 group. Large tumor size (5 cm) is significantly associated with poor 5-year and 10-year overall survival.
Conclusion
Survival outcomes of patients with a harvest of ≥25 nodes were not significantly different compared with the <25 group in stage II colon cancer with no risk.
INTRODUCTION
Colon cancer is the third most common malignant disease in the world with an estimated 1.1 million cases diagnosed in 2020 [1]. Despite a substantial decline in its global incidence, colon cancer remains the second most common cause of cancer-related death each year [1]. Moreover, while the rate of positive oncologic outcomes is rising due to rapid advances in chemotherapy, radiotherapy, and immunotherapy, the primary appropriate approach to colon cancer treatment is still colectomy with lymphadenectomy. There are two main components that should be considered for effective lymphadenectomy. First, the extent of lymph node resection should be considered. After the introduction of complete mesocolic excision (CME) and D3 dissection in the mid-2000s, numerous surgical units around the world implemented these techniques for lymphadenectomy in colon cancer surgery [2]. Although distinct technical differences exist, the overall purpose of CME and D3 dissection is similar, which is to remove all lymphovascular fatty tissue (mesocolon) surrounding the colon. Both CME and D3 dissection depend on two main components: complete dissection following embryological anatomical planes and high tie vascular ligation [3]. The second point to consider is the impact of lymph node yield (LNY). It is well-known that the number of metastatic lymph nodes is a key factor for survival of patients with colon cancer [4].
There have been multiple studies addressing the optimal number of harvested lymph nodes in colon cancer [5,6]. Until the mid-2000s, different studies suggested a cutoff value that varied from 6 to 21 [5,7]. The number 12 was first suggested in 1990 by the World Congress of Gastroenterology and has been widely used since that time [8]. Recent guidelines, including those of the American Joint Committee on Cancer (AJCC), the National Comprehensive Cancer Network (NCCN), and the American Society of Clinical Oncology (ASCO) recommend that more than 12 nodes should be removed [9].
To date, few studies have investigated lymph node cut values of more than 20. However, it is unclear whether the current guidelines reflect the recent findings on the effectiveness of the resection of an increased number of lymph nodes. The aim of this study was to assess the impact of an LNY of more than 25 on survival in right-sided colon cancer stage II with no risk factors.
METHODS
Patients
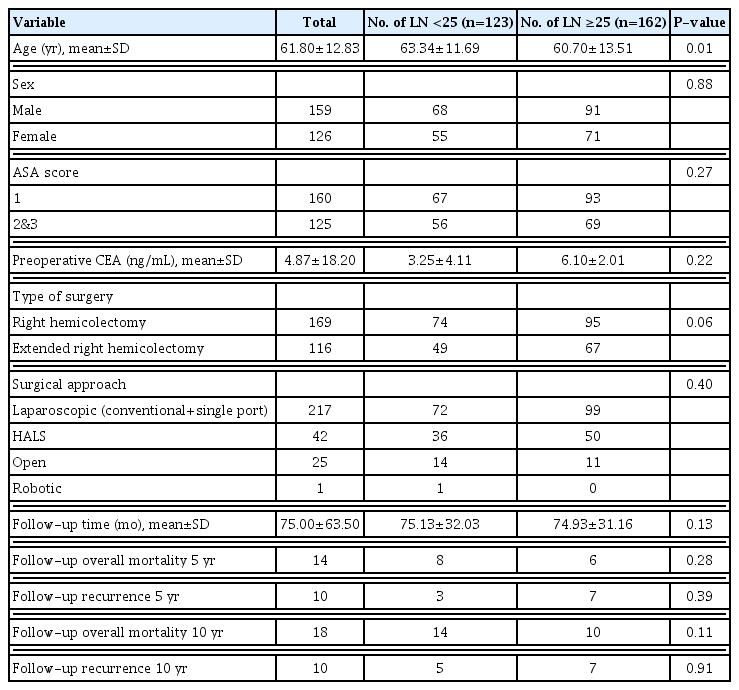
We included 285 patients who underwent a right or extended right hemicolectomy with D3 lymphadenectomy in stage II colon cancer (according to the 6th AJCC) during the period from January 2010 through December 2015. In this retrospective study, D3 dissection was mandatory in all patients who underwent elective right or extended right hemicolectomy. Surgeons in the unit followed a standardized operative approach for D3 dissection. Surgeons in the unit followed a standardized operative approach for D3 dissection. All patients had pathologically confirmed adenocarcinoma or mucinous adenocarcinoma, with tumor grade categorized as well differentiated, moderately differentiated, poorly differentiated, or undifferentiated. Colon cancer was evaluated as right-sided colon cancer including cecum, ascending colon, hepatic flexure, and transverse colon. The number of harvested lymph nodes was evaluated and recorded.
Exclusion criteria were as follows: double primary cancer, severe comorbidity as judged by an American Society of Anesthesiologists score of 4, patients who underwent an emergency operation, surgery with palliative intent, history of another malignancy, history of transplantation surgery, or colonic perforation or obstruction.
All patients had routine follow-up every 3 to 6 months for 10 years postoperatively with serial measurement of serum tumor markers including carbohydrate antigen 19-9 and carcinoembryonic antigen. Abdominopelvic and chest computed tomography scans were performed annually in addition to colonoscopy as deemed clinically appropriate. Follow-up was defined as the time from the date of primary surgery to a patient event, such as disease recurrence or death. There was no minimum duration of follow-up.
The study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC 2021-01-111-001) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived because this study design is a retrospective review.
Lymph node and microsatellite status evaluation
After resection of the colon, specimens were fixed overnight in 10% formalin for at least 24 hours. After fixation, the mesenteric adipose tissue was separated into thin slices (4 μm thick) and lymph nodes were sampled. After conventional histological staining with hematoxylin and eosin, the lymph nodes were microscopically inspected for the presence of metastasis. To analyze microsatellite instability (MSI), DNA was extracted from the tumor and from paraffin-embedded paired normal tissue. Two mononucleotide repeat markers (BAT25 and BAT26) and three dinucleotide markers (D5S346, D2S123, and D17S250) were used to determine microsatellite status following amplification using the polymerase chain reaction [10]. MSI-high was defined as instability at two or more markers. MSI-low was defined as only one marker exhibiting a shifted allele. The rest were classified as microsatellite stable (MSS).
Statistical analysis
The three main points of interest consist of the LNY per patient, recurrence of disease, and patient death. Patients were separated into two groups according to the number of harvested lymph nodes as follows: lymph node numbers from 0 to 24 and lymph node numbers ≥25. Patients were also grouped according to size of tumor into larger tumor (≥5 cm) and smaller tumor (<5 cm) groups. Overall survival (OS) was defined as the interval between the diagnosis date and time of death for any reason or the last follow-up. Disease-free survival (DFS) was measured from the date of surgery to the date of recurrence. Kaplan-Meier analysis was used to estimate the survival difference between the different subgroups. After surgery for the primary tumor, patients who developed local recurrence or metastasis were no longer considered disease free for statistical analysis. Patients who died at any time, for any reason, after surgery were counted as deaths in OS analysis. The chi-square test (Fisher test in small samples) was used to compare two qualitative samples in univariate analysis. Multivariate Cox proportional hazard model was used to evaluate the relationship between patient characteristics, tumor size, LNY, and survival including DFS and OS. Two-sided P<0.05 was considered statistically significant. All statistical analyses were performed using a standard software package (Stata, version 16.0; StataCorp, College Station, TX, USA).
RESULTS
A total of 1,992 patients who underwent right or extended right hemicolectomy in stage II colon cancer between 2010 and 2015 were identified in our hospital. Of these, 1,627 patients were excluded due to having risk factors, including lymphovascular or perineural invasion. Sixty-one patients were excluded due to obstruction by cancer. A total of 1,707 patients were excluded from our study because they met the exclusion criteria. Finally, 285 patients with no risk factors remained eligible for analysis. Of these, 169 patients (59.3%) underwent right hemicolectomy and 116 patients (40.7%) underwent extended right hemicolectomy. The median follow-up period and range was 65 months. Median age at diagnosis was 61.8 years, standard deviation (SD) 12.8 years. A total of 55.7% of patients were male. There were no significant differences as regards age, sex, body mass index (BMI), and other variables. The baseline characteristics of patients who underwent right hemicolectomy are summarized in Table 1.
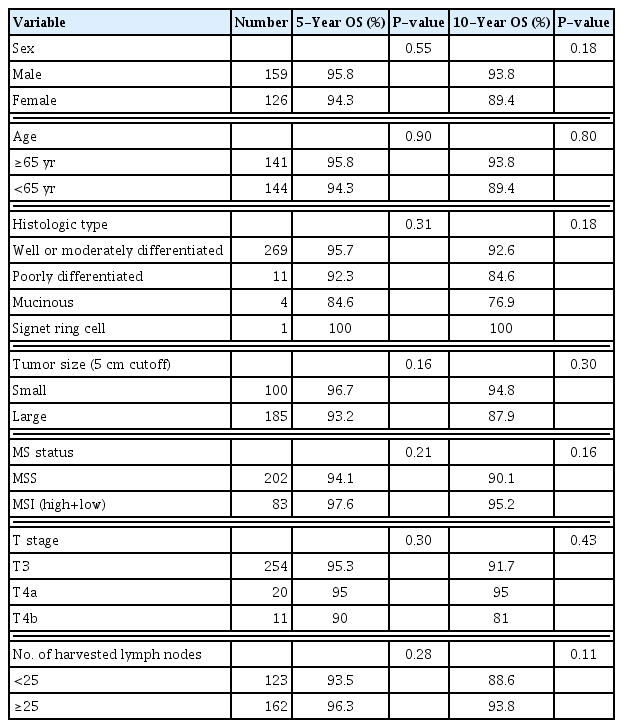
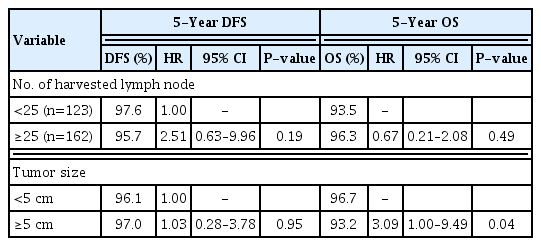
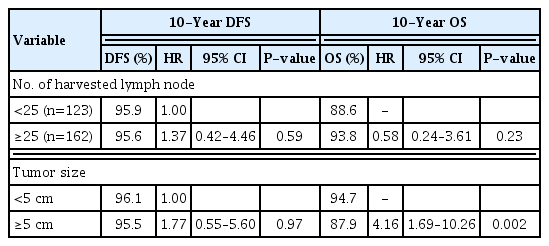
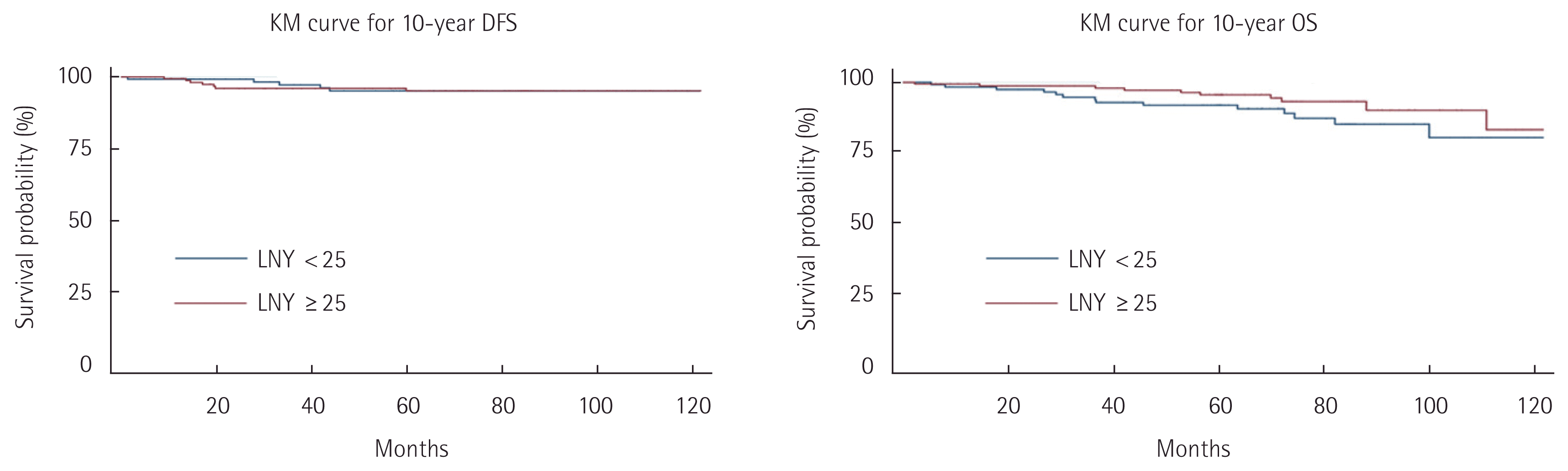
Table 2 shows the pathologic and genetic characteristics of the patients. Moderately differentiated adenocarcinoma was the predominant histology, and the majority of the tumors were located in the ascending colon including the hepatic flexure. The median LNY was 27.4 (SD 11). Although the median value was 27.4, the cutoff value was set at 25 to have the sample size as similar as possible between the two groups. A total of 162 patients (56.8%) had an LNY ≥25. Compared with the small tumor size group, the large tumor size group was significantly associated with LNY ≥25. LNY was significantly lower in patients with MSI (P<0.001). Univariate analyses were performed to identify clinicopathological factors that can predict 5- and 10-year OS and DFS. There was no significant difference in survival between the subgroups (Tables 3, 4). Multivariate analysis, adjusted for gender, age, BMI, and tumor size, showed significantly higher risk of 5-year OS (hazard ratio [HR], 3.09; 95% confidence interval [CI], 1.00–9.49) and 10-year OS (HR, 4.16; 95% CI, 1.69–10.26) in the large tumor size group (Tables 5, 6). There was no significant difference in both 5- and 10-year OS and DFS between LNY <25 and ≥25 groups (Figs. 1, 2).

Five-year disease-free survival (DFS) and overall survival (OS) rates of patients with stage II colon cancer without risk factors. LNY, lymph node yield; KM, Kaplan-Meier.
DISCUSSION
The results of this study showed that stage II right-sided colon cancer with no risk factors, even if the number of nodes was more than 25, was not related to survival. Since the 1990s, many studies have shown that LNY is associated with patient survival [4,6]. Additionally, LNY can be an index to quality of surgery and also a factor in deciding whether to use adjuvant chemotherapy [6]. However, the impact of total number of harvested nodes is still debated. Existing research suggests that harvesting more lymph nodes is an independent factor for predicting survival [6,7]. Other studies suggest harvesting of more lymph nodes provides high numbers of lymph nodes for investigation, which is associated with an upstaging effect (the Will Rogers phenomenon) [11]. For this reason, numerous studies to determine a “cutoff value” for the number of nodes to be harvested would seem to be appropriate. In the early 1990s, the working party report in the World Congress of Gastroenterology argued that at least 12 nodes should be harvested [8]. On the other hand, in 1994, Hernanz et al. [12] insisted that at least six lymph nodes should be harvested for accurate staging. Until the mid-2000s, guidelines including those of the AJCC and the International Union Against Cancer recommended obtaining at least 7 to 14 lymph nodes in radical resections [13]. In recent years, AJCC, ASCO, and NCCN guidelines recommended that at least 12 or more lymph nodes should be harvested as a standard to maximize survival.
However, it is unclear whether these guidelines adequately reflect recent findings on the number of harvested lymph nodes in colon cancer surgery. According to a recent study, the LNY in colon cancer surgery in the last 20 years has tended to increase from 14.91 to 21.38 [14]. This increase is thought to be due to a number of factors. First, thanks to newly developed surgical techniques, the global extent of lymphadenectomy has widened. In 2009, CME became the standard surgical procedure in Western countries [2]. In addition, D3 lymph node dissection is the minimum recommended for stage II or III colon cancer [3]. Before the era of these techniques, D2 dissection and conventional non-CME were performed worldwide. At that time, although the concept of D3 lymph node dissection was reported by Toyota, he did not strongly advocate for extended dissection [15]. Second, node retrieval techniques were also developed. Traditionally, lymph node dissection from surgical specimens relied completely on manual examination (Quirke method) and visual inspection [16]. Therefore, the number of detected nodes was largely dependent on the diligence, skill, and patience of pathologists [17]. Recently, fat clearance methods using procedures involving such things as glacial acetic acid and ethanol have been developed [18]. Previous studies suggested that LNY increased from 15 to 23 (P=0.02) with the introduction of the alcohol fat clearance technique [19]. As a result, while the development of surgical techniques for general surgeons has progressed, the development of national guidelines for pathologists has also progressively standardized practices [20].
A recent study showed that LNY of 20 or more was associated with better survival outcomes [21]. However, in this study there was no significant difference between LNY <25 and ≥25 groups in 5- and 10-year OS and DFS. Our study found that harvesting between 12 and 25 lymph nodes is sufficient to establish reliability in metastatic lymph node assessment.
Several studies investigated the change of node positivity as a result of increasing LNY [6]. Goldstein [6] reported similar findings in 1996 and 2002, analyzing 2,427 cases in the Surveillance, Epidemiology, and End Results database and 750 cases in a systematic review. In those studies, although the number of harvested lymph nodes increased from 3.3 in the 1950s to 19.4 in the 1990s, the authors observed that the number of metastatic nodes barely increased even if more than 17 lymph nodes were harvested. Similarly, a recent study suggests that sampling beyond a certain number of lymph nodes is not associated with an increased rate of node-positive status [22]. Another recent study suggests that a node harvest of only seven is equivalent to those with 30 nodes when analyzing survival outcomes in pT3 colon carcinoma [23]. The results obtained in our study confirm previous findings that LNY over 17 is not associated with a better survival outcome.
It is well-known that tumor size is a predictor of LNY [24]. It is postulated that a more intense antigenic immune response to the draining lymph node occurs in large tumors [25]. Tumor size also impacts significantly on survival in colon cancer, as several other studies have demonstrated [24]. This is more commonly due to iron deficiency anemia in larger tumors with more aggressive tumor biology and tumor necrosis [24]. The findings of this study confirm previous findings that larger tumor size (>5 cm) is associated with poor 5-year OS and DFS. Interestingly, in our study we observed a significant association between tumor size and survival in 5- and 10-year OS but not DFS. Similarly, Dai et al. [26] suggested that tumor size is an independent factor only for OS in a multivariate analysis of 4,057 patients in 2017.
Before proposing a potential hypothesis to explain these findings, several confounders need to be taken into accounts. First, it can be assumed that although recurrence occurred, members of the small tumor size group survived longer. There is little research on whether the tumor size at first surgery influences chemotherapy in recurrent colon cancer. In our opinion, further research is necessary to answer this question. Second, our research used data from the Korean National Health Insurance Service and Statistics Korea to measure mortality. In the case of patients who died from causes other than cancer, it was difficult to accurately determine whether the cause of death was related to colon cancer. Due to this potential bias of data in our study, more research is required to explain the inconsistent discrepancy between DFS and OS.
We also examined the MSI status of right-sided cancers. MSI analysis of the 225 colon cancers revealed 83 (29.12%) cases of MSI-high cancer. It is well-known that MSI tumors are located predominantly in the right colon and tend to appear poorly differentiated [27]. While MSI tumors are known to have a less aggressive course than MSS tumors, MSI tumors selectively demonstrated highly upregulated expression of multiple immune checkpoints including five inhibitory receptors: PD-1, PD-L1, CTLA-4, LAG-3, and ID [28]. In the present study, we found a higher LNY in the MSI tumors than in the MSS tumors. This finding confirms the observations of earlier studies [29]. In 2012, Belt et al. [30] suggested that high LNY (≥10) was significantly associated with the MSI phenotype (high LNY: 26.3% MSI tumors vs. low LNY: 15.1% MSI tumors; P=0.01). In our current study, high LNY was significantly associated with MSI tumors even when the cutoff value was raised to 25.
To the best of our knowledge, the present study is the first to explore the significance of a cutoff value of 25 in colon cancer. Recent studies report outcomes that are similar to our study [29]. Our study extended these reports by considering multiple variables. Patients with risk factors including lymphovascular and perineural invasion were excluded and we also assessed MSI to reduce variability in the study population.
There are several limitations to the current study. First, these data are retrospective and there may be inherent biases which we attempted to control. Second, the surgeries were performed by many surgeons and the pathologic examination involved many pathologists. Third, as noted above, this research used data from Statistics Korea and the Korean National Health Insurance Service to measure mortality. It is possible that we had a measurement error when a person with colon cancer died of non-cancer disease.
In conclusion, survival outcomes of patients with a harvest of more than 25 nodes were not significantly different compared with the <25 group in stage II colon cancer with no risk factors. Nonetheless, the findings of our study should not compel surgeons to maximize LNY. These findings should be validated in other stages of colon cancer in larger prospective studies.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.