Risk factors for atypical lymph node metastasis in gastric cancer
Article information
Abstract
Purpose
The present study aimed to evaluate atypical lymph node metastasis rates according to tumor depth, size, and location in patients with gastric cancer.
Methods
A total of 727 gastric adenocarcinoma patients, with metastasis to 1 or 2 lymph nodes, who underwent radical gastrectomy with curative intent from May 2003 to May 2017, were enrolled in this study. The characteristics of atypical (skip or transversal) metastases were evaluated according to the following risk factors: longitudinal versus circumferential location, size, and T stage of the tumor.
Results
The rates of skip and transversal metastases were 8.4% and 15.5%, respectively. Skip metastases were present throughout, regardless of the primary tumor location. On the contrary, transversal metastases of gastric cancer were most frequently observed in the lower third region (19.5%, P=0.002). When the size of the tumor is large (>4 cm), transversal metastasis was found to be significantly more common (P=0.035), compared with skip metastasis, which was less common (P=0.011). There was no significant correlation between atypical metastases and tumor depth.
Conclusion
Lower and larger tumors were more likely to have transversal metastases compared with others; however, skip metastases were less common in large tumors.
INTRODUCTION
Lymph node (LN) metastasis is very important for the prognosis of gastric cancer, and topics surrounding the amount of LN dissection during surgery remain controversial. Nodal metastasis of gastric cancer cells is relatively more aggressive than other cancers and removal is necessary even for micrometastasis nodes [1].
Currently, D2 dissection is accepted as the gold standard of gastric cancer treatment. However, D1 dissection is also performed in early gastric cancer (EGC) without LN metastasis in Korea and Japan [2,3]. Endoscopic mucosal resection, endoscopic submucosal dissection, and sentinel node navigation surgery (SNNS) have been attempted to preserve gastric function and improve quality of life according to the recent minimal invasive tendency [4–8].
Gastric cancer has a relatively complex multidirectional lymphatic flow, and the rate of skip metastasis is not low, making it difficult to apply SNNS in the treatment of gastric cancer [9–11]. Therefore, if risk factors related to the possibility of atypical LN metastasis, such as skip metastasis, are identified, it is helpful to set an indication of minimally invasive surgery.
To date, skip metastases have been studied several times; however, to the best of our knowledge, studies on transversal metastases are rare [12,13]. There were also only a few studies on atypical LN metastasis in advanced gastric cancer (AGC). The purpose of this study was to determine the distribution of atypical LN metastasis according to tumor location and depth.
METHODS
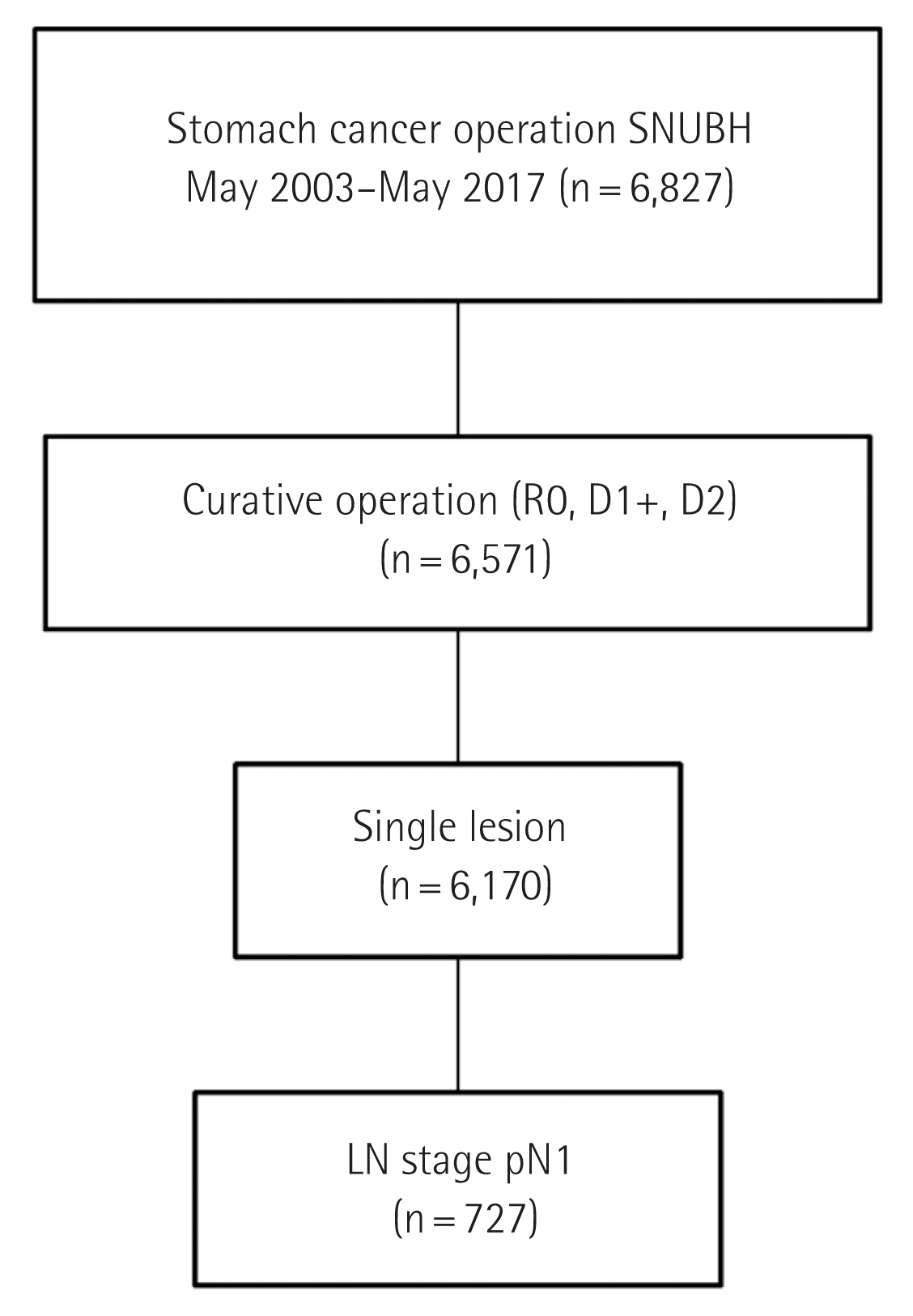
This study was approved by Institutional Review Board of the Seoul National University Bundang Hospital (IRB No: B-2001/586-110). Consecutive gastric tissue specimens were obtained retrospectively from 6,827 patients who had undergone curative resection for gastric cancer at Seoul National University Bundang Hospital (Seongnam, Korea) between May 2003 and May 2017. Palliative resection, R1 or R2 resection, and multiple lesions were excluded from the study; only tumors in stage pN1 were selected. Therefore, a total of 727 patients were selected for this study (Fig. 1).
Medical charts and pathology reports were reviewed to obtain clinical and pathologic data, including age, sex, pathological TNM staging according to the American Joint Committee on Cancer 7th edition, depth of invasion, angiolymphatic invasion, neural invasion, and histologic subtype.
Skip metastases and transversal metastases were considered as types of atypical LN metastases. Skip metastasis was defined as the presence of a metastatic LN in an extraperigastric area without perigastric involvement. Perigastric LNs were LNs numbered 1 to 6. Extraperigastric LNs were the remaining ones. Transversal metastasis was defined as metastasis to the opposite side of the tumor location, without metastasis to the tumor side.
Statistical analysis was performed by SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Descriptive data were presented as the mean±standard deviation. Chi-square test and Fisher exact test were used for comparisons between the two groups. Risk factors that independently influenced skip or transversal metastases were determined by logistic regression analysis.
RESULTS
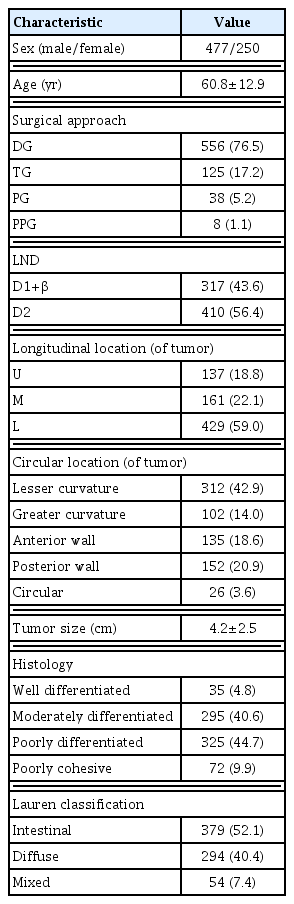
The characteristics of the patient groups are shown in Table 1. Four hundred seventy-seven (65.5%) patients were male. Distal gastrectomy (76.5%) and total gastrectomy (17.2%) were the most common. The longitudinal location of tumor was greatest in the lower third region (59.0%), followed by middle third region (22.1%), and upper third region (18.8%). Tumor’s circumferential location was most common in the lesser curvature (42.9%), followed by posterior wall (20.9%), anterior wall (18.6%), and greater curvature (14.0%). Histologically, most cases were diagnosed as well differentiated (4.8%), moderately differentiated (40.6%), poorly differentiated (44.7%), or poorly cohesive (9.9%), which were relatively rare. Single node metastasis was found in 470 patients (64.6%), and two-node metastases were found in 257 patients (35.4%).
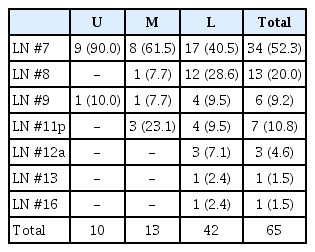
The LN station with the most skip metastasis was LN #7 (Table 2). Especially, as the longitudinal location of the tumor was higher, skip metastasis was more likely to be LN #7. The next most common metastatic site was LN #8, followed by #9, #11p, and #12a.
In the univariate and multivariate analyses, size of tumor was significantly correlated with skip metastasis (Table 3). The skip metastasis rate was significantly lower when tumor size was more than 4 cm or the circumferential location of the tumor was in the greater curvature. On the other hand, both longitudinal locations and size of tumor were associated with transversal metastases (Table 4). Transversal metastasis was significantly higher when the tumor position was located in the lower third region, or when the tumor size was greater than 4 cm.
Information of the T classification of enrolled patients and the number of LN metastasis is in Supplementary Tables S1 and S2.
DISCUSSION
The results of the present study suggest that atypical LN metastasis may not be particularly more prevalent in AGC compared with EGC. The results presented in this study are similar with those of previous reports [14,15]. Specifically, there was a decrease in skip metastasis and an increase in transversal metastasis in large tumors (size >4 cm).
One of the greatest stumbling blocks in the application of SNNS is atypical metastasis, which skips the sentinel nodes. Low rate of atypical LN metastasis means cancer spreads in order from near to far, step by step. Then it may be more feasible to apply SNNS to AGC, since skip metastases are significantly decreased in AGC, as shown in this study. More studies are needed for the application of SNNS in AGC.
The most common sites at which skip metastases were observed were LNs #7 and #8. This is consistent with the findings of earlier studies [10,12,15–17]. This suggests that perigastric lymphatic flow may be directly connected to LN #7 and #8. LN #7 and #8 are classified as extraperigastric LNs, but they are close to the perigastric LN; therefore, it is reasonable to consider including LN #8 in D1+ dissection, as well as LN #7 in D1 dissection.
There are several hypotheses explaining the occurrence of atypical metastasis. The first is an abnormal lymphatic flow. When sentinel node biopsy is performed, it is reported that the sentinel LN may be found in the extraperigastric region and nodal metastasis can be observed in those skipping sentinel LNs [6]. There is also a view that lymphatic flow around the posterior gastric artery may be a route for skip metastasis [18]. Skip metastases being common at LNs #7 and #8 suggests that atypical lymphatic flow leading to those LN stations may be frequent. It was a reasonable decision to include node #7, which had a high frequency of skip metastases, as part of D1 dissection in the Japanese Gastric Cancer treatment guidelines [19]. It is more like a perigastric LN than an extraperigastric LN.
The second hypothesis is that a host-related immunological defense mechanism is likely to result in skip metastasis due to the disappearance of primary sentinel LN tumor cells. This mechanism has been suggested because the presence of micrometastasis in EGC does not affect the prognosis [20].
Other possibilities may be that cancer cells bypass the sentinel LN and metastasize to the remote LN stations [14].
In this study, we limited the number of metastatic LNs to one or two. If positive metastatic LNs are too many, it is difficult to predict the lymphatic flow. To clarify the LN station in which the first or second atypical metastasis occurred, the target tumor stage of this study was limited to the N1 stage.
The skip metastasis rate was significantly lower when the tumor was located circumferentially in the greater curvature. This is probably because the distance between the metastatic LN and the tumor is much longer when the tumor location is in the greater curvature compared with cases where the tumor location is the lesser curvature.
The present study has some limitations. Despite the large data, it is obtained from a single institution. Secondly, due to the retrospective nature of this study, some bias of sources may be inevitable.
In conclusion, large tumors and tumors located in the lower third region were more likely to have transversal metastases than others, but skip metastasis was observed less frequently in large tumors. Tumor depth was not associated with atypical metastasis. Caution should be taken when performing SNNS for tumors located in the lower part of the stomach, or for large tumors, but SNNS might be considered in AGC as well as in EGC.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Supplementary Information
The numbers of positive LN according to T stage in gastric cancer
Supplementary data can be found: https://doi.org/10.14216/kjco.19018.S1
The portions of N stage according to tumor advance
Supplementary data can be found: https://doi.org/10.14216/kjco.19018.S2