Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in management of peritoneal carcinomatosis: Single center experience in Korea
Article information
Abstract
Purpose
Peritoneal carcinomatosis (PC) has been considered a terminal condition and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIEPC) is regarded as an alternative therapeutic option. This study aimed to evaluate the 30-day clinical outcomes of CRS/HIPEC and the feasibility of the surgery by investigating the morbidity and mortality in Inje University Hospital.
Methods
Data were retrospectively collected from 19 patients with PC who underwent CRS/HIPEC at Inje University Hospital in 2018. We evaluated pre-, intra-operative parameters and postoperative clinical outcomes and early complications.
Results
The mean operating time was 506.95 minutes and the mean blood loss was 837.11 mL. Six cases (31.58%) had morbidity of grade III or above. A longer operating time (≥560 minutes, P=0.038) and large blood loss (≥700 mL, P=0.060) were positively correlated with grade III or worse postoperative complications.
Conclusion
Our early experience with CRS/HIPEC resulted in a 31.58% morbidity rate of grade III and above, with risk factors being longer operating time and greater intraoperative blood loss. As the surgical team’s skills improve, a shorter operating time with less intraoperative blood loss could result in better short-term outcomes of CRS/HIPEC.
INTRODUCTION
Peritoneal carcinomatosis (PC) can result from either the direct dissemination of gastrointestinal and gynecological cancers or secondary metastasis along the peritoneal surface into the abdominal cavity [1–3]. In patients with a peritoneal metastasis only, cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is a potential curative option [4–6]. The basic concept of CRS/HIPEC involves first removing all macroscopic tumors and then delivering hyperthermic anticancer drugs to the microscopic residual tumor cells [7]. Verwaal et al. [8,9] performed a randomized controlled trial with 105 colorectal PC patients, with the median progression-free survival and the median disease-specific survival of 12.6 months and 22.2 months, respectively, in the CRS/HIPEC group, showing better survival than in the systemic chemotherapy only group. Furthermore, there are numerous reports of positive results of CRS/HIPEC in patients with PC [10,11]. Nevertheless, many surgeons are still concerned about the high morbidity and mortality of CRS/HIPEC. A number of reports have shown a 1.1%–4.8% mortality rate and 29.8%–43% grade III/IV morbidity rate [10,12–14]. Thus, in this study, we would like to evaluate the 30-day clinical outcomes of CRS/HIPEC and the feasibility of the surgery by investigating the morbidity and mortality in our institution.
METHODS
Data were retrospectively collected from patients with PC who underwent CRS/HIPEC at Inje University Hospital from January 2018 to December 2018. Individuals older than 80 years, with Eastern Cooperative Oncology Group (ECOG) ≥3, or with extra-abdominal metastasis were excluded. Patients with stage III epithelial ovarian cancer, or peritoneal carcinomatosis of colorectal cancer or pseudomyxoma peritonei without the exclusion criteria were included. The Institutional Review Board of Inje University Hospital, approved this study (IRB No. 19–0025).
Evaluation parameters
Preoperatively, comorbidities and the American Society of Anesthesiologists (ASA) score were evaluated in all patients. The preoperative peritoneal carcinomatosis index (PCI) score was assessed using abdominopelvic and chest computed tomography (CT), positron emission tomography-CT (PET-CT), and previous diagnostic laparoscopy as available. The intraoperative PCI score (Fig. 1) [15] was assessed using the methods of Jacquet and Sugarbaker [16], which divides the abdominopelvic cavity into 13 regions and each region is graded using the following scale: 0 points, no tumor; 1 point, tumor <0.5 cm; 2 points, tumor 0.5–5 cm; 3 points, tumor >5 cm. Completeness of cytoreduction (CC) was assessed using the diameter of the remnant tumor: CC-0, complete removal of visible tumor; CC-1, remnant tumor <0.25 cm; CC-2, residual tumor 0.25–2.5 cm; and CC-3, visible tumor >2.5 cm.
Postoperative surgical complications were classified using the Clavien-Dindo classification. The postoperative length of stay in the intensive care unit (ICU), length of hospital stays, ICU readmission, postoperative day of first flatus, postoperative day of starting diet, and complication-related readmission after discharge were also evaluated.
CRS/HIPEC procedure
All patients routinely underwent preoperative bowel preparation. CRS was performed to remove the primary tumor, metastatic lymph nodes, and all intraperitoneal metastases. Parietal peritonectomy and visceral resections were performed using the Sugarbaker technique [17,18]. In patients with either an unresectable mass or severe small and large bowel mesenteric seeding, we used a high-voltage monopolar device for cauterization. HIPEC was performed using open and coliseum techniques. First, 3 L heated perfusion solution (Physionel, 1.5% dextrose peritoneal dialysis solution) was infused intraperitoneally at 800–1,000 mL/min using a Belmont hyperthermic pump. When the intraperitoneal fluid reached 40°C–41°C, paclitaxel 87.5 mg/m2 was injected initially, followed by 43.75 mg/m2 at 30 and 60 minutes for ovarian cancer; for other cancers, including colorectal cancer, mitomycin-C 17.5 mg/m2 was injected initially followed by 8.75 mg/m2 at 30 and 60 minutes. The temperature of the perfusate was maintained at 41°C–42°C by monitoring the temperature using pelvic and subphrenic thermometers. Bowel anastomosis, if needed, was performed after HIPEC, and three closed drains were placed in every patient, in the pelvic, right subphrenic, and left subphrenic areas.
Statistical analyses
We conducted logistic regression to identify risk factors associated with complications. A P-value less than 0.05 was deemed statistically significant. The analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics and perioperative outcomes
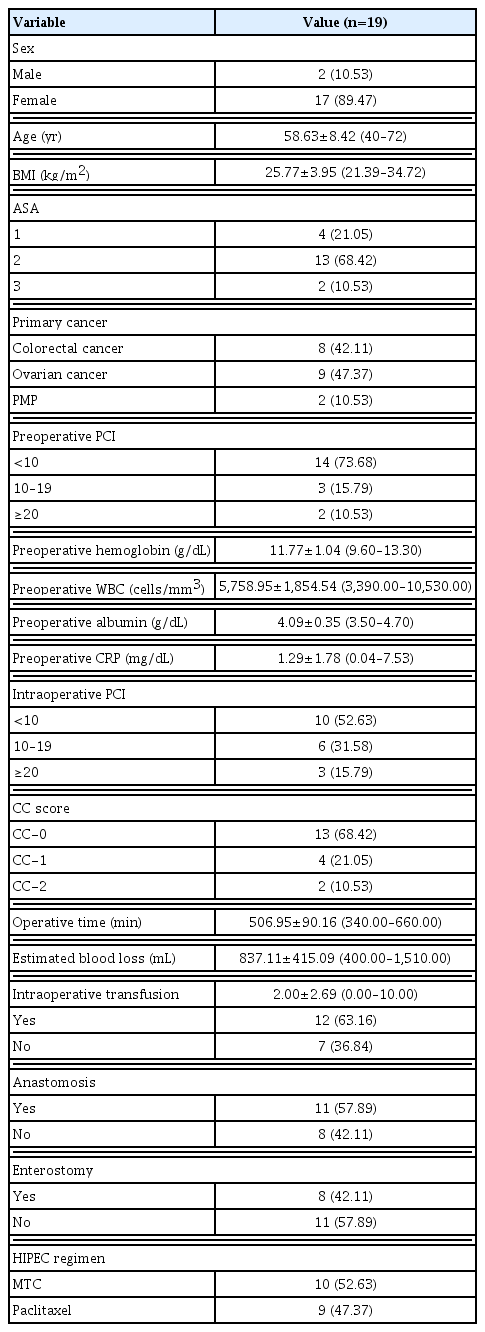
Nineteen patients with colorectal cancer (n=8), ovarian cancer (n=9), and pseudomyxoma peritonei (n=2) underwent CRS/HIPEC during the study period. Their mean age was 58.63 years and 89.47% were female. The mean body mass index (BMI) of the patients was 25.77±3.95 kg/m2 (range, 21.39–34.72 kg/m2). The ASA score was <3 in 17 patients.
The median PCI score was 9 (range, 3–39), and 10.53 (2/19) had a PCI greater than 20. CC-0 and 1 was attained in 89.47% (n=17). The mean operating time was 506.95 minutes (range, 340–660 minutes) and the mean blood loss was 837.11 mL (range, 400–1,510 mL). Intraoperative transfusion was performed in 12 patients (63.16%). Bowel anastomosis was performed in 11 patients (57.89%) and enterostomy in eight patients (42.11%). We used paclitaxel to treat ovarian cancer (47.37%) and mitomycin-C for colorectal cancer and pseudomyxoma peritonei (52.63%) (Table 1). The average ICU and hospital stays were 2.26 and 21.42 days, respectively. Patients began sipping water at 4.00 postoperative days and a soft diet at 5.57 postoperative days. Active ambulation was encouraged from 4.11 days postoperatively and the first flatus was at 4.58 postoperative days. Complete removal of the closed drains was done at 12.45 days. Eight patients had packed red blood cell transfusions after surgery. One patient was re-admitted to the ICU with aspiration pneumonia; the patient ultimately died (Table 2).
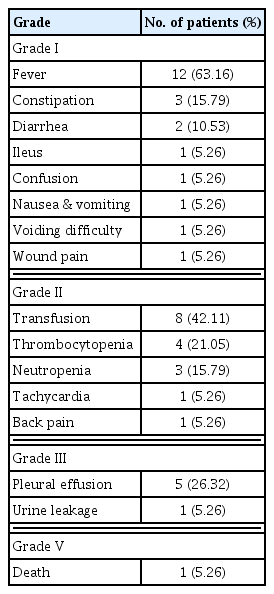
Postoperative complications according to the Clavien-Dindo classification
Among grade I postoperative complications, fever was the most common (63.16%). Common grade II complications were hematological abnormalities, such as anemia, neutropenia, and thrombocytopenia. Regarding grade III complications, six patients underwent interventional therapies for pleural effusion or urine leakage. No patients had to undergo reoperation under general anesthesia. One patient with pleural effusion had a grade V complication; this patient was asphyxiated while undergoing interventional therapy to relieve a pleural effusion (Table 3).
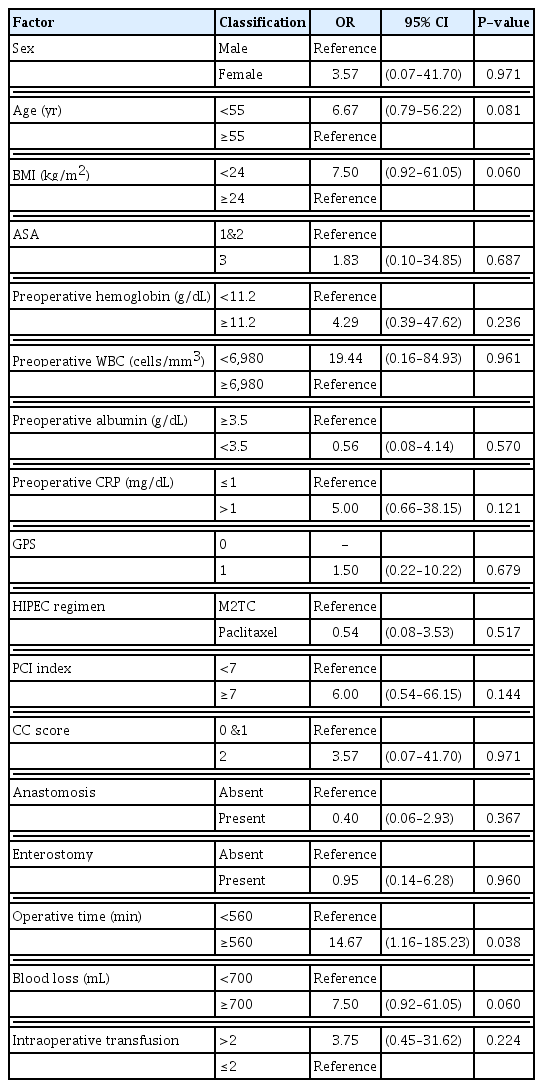
Longer operating time (≥560 minutes, P=0.038) had statistical significance, and large blood loss (≥700 mL, P=0.060) had a tendency of positive correlation with grade III or worse postoperative complications (Table 4).
DISCUSSION
Historically, PC of gynecological and gastrointestinal malignancies was considered a terminal condition and the only therapy was palliative chemotherapy. In the 1990s, Sugarbaker [17,19] introduced CRS/HIPEC as an alternative therapeutic option in PC. However, many surgical and medical oncologists still doubt the effects of CRS/HIPEC on the oncological prognosis, as the surgical morbidity and mortality remain high; thus, it is still not a popular means of managing PC.
Our postoperative clinical outcomes were similar to those of other CRS/HIPEC studies. Park et al. [20] reported their experience with 66 patients with appendiceal and colorectal cancer with PC. The mean hospital stay was 20.2 days (range, 8–70 days), mean time to first sipping water was 4.7 days (range, 1–21 days), mean time to first soft diet was 9.5 days (range, 3–4 days), and mean time to Jackson-Pratt drain removal was 13.4 days (range, 5–43 days). Our results are in line with these values. Another study reported comparable data [21].
A systemic review of CRS/HIPEC in PC of colorectal origin reported a perioperative morbidity rate of 14.8%–57% and mortality rate of 0%–12.0% [22]. Another systemic review of CRS/HIPEC for malignancy of varying origins reported 0%–52% postoperative morbidity, the most common complications being anastomotic leakage, venous thromboembolism, and hematologic toxicity [23]. In this study, we categorized the postoperative complications using the Clavien-Dindo classification. The most common grade I complication was fever. Patients mostly had fever within three postoperative days; this is probably due to atelectasis from the long operation or postoperative ventilator care. Among grade II complications, the most common were hematological abnormalities such as anemia, neutropenia, or thrombocytopenia. This was common at 5 to 7 postoperative days, and was corrected by transfusion or hematopoietic agents. Six cases (31.58%) had morbidity of grade III or above. Although our study appeared to have a higher overall morbidity rate than other studies, this is probably because we considered various complications that the other studies did not include. Notably, our grade III and above morbidity rate was similar to that of other studies [10,12–14]. Fortunately, there were no major complications such as bleeding or anastomotic leakage requiring reoperation in our study, and only five patients needed interventional therapy for pleural effusion.
Examining 76 patients with CRS/HIPEC, Arakelian et al. [24] noted that the PCI and ASA scores predicted pleural effusion. Our five patients with pleural effusions all had very severe diaphragmatic PC needing extensive diaphragmatic peritonectomy. Therefore, patients undergoing diaphragmatic peritonectomy require meticulous postoperative pulmonary monitoring and care.
Many studies have reported that a longer operating time is a risk factor for postoperative complications (infectious complications, sepsis, and pulmonary complications) [25–29]. Daley et al. [25] noted that in cases with long operating times, there were increases in infectious complications, deep vein thrombosis, and wound disruption; for every 1,000 cases, there were 116 complications per operating room hour. We also found that a longer operating time was a risk factor for grade III complications (odds ratio, 14.67; P=0.038). Many factors affect the operating time, such as the patient’s BMI, number of organs resected, prior surgery, and the surgical team’s experience. Another factor with a tendency to contribute to an increase in complications was a large intraoperative blood loss (odds ratio, 7.50; P=0.060). In a study of 1554 colorectal cancer patients, Okamura et al. [30] found that intraoperative blood loss has a detrimental effect on morbidity; blood loss >200 mL (46%) had a higher morbidity rate than blood loss <200 mL (30%).
This study was limited by its small sample size and retrospective nature. However, it has value in reporting our early experience with CRS/HIPEC in a single center in Korea, with a frank description of the short-term clinical outcomes.
In conclusion, our early experience with CRS/HIPEC resulted in a 31.58% morbidity rate of grade III and above, with risk factors being longer operating time and greater intraoperative blood loss. As the surgical team’s skills improve, a shorter operating time with less intraoperative blood loss could result in better short-term outcomes of CRS/HIPEC.
ACKNOWLEDGMENTS
We would like to thank Nr. Mi Kyung Jung, Kum Ryun Chun, Ho Jung Jo, and Dan Bi Heo for participating in this study.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.