Clinicopathologic characteristics and prognosis of remnant gastric cancer
Article information
Abstract
Purpose
Remnant gastric cancer is defined as a malignant tumor developing on the remnant side of stomach after partial gastrectomy. The purpose of this study is to evaluate the clinical characteristics and prognosis of remnant gastric cancer according to the cause and the reconstruction method of previous surgery.
Methods
Between January 2007 and February 2016, we analyzed 39 patients with their medical records who were diagnosed as remnant gastric cancer and underwent gastrectomy at Inje University Busan Paik Hospital.
Results
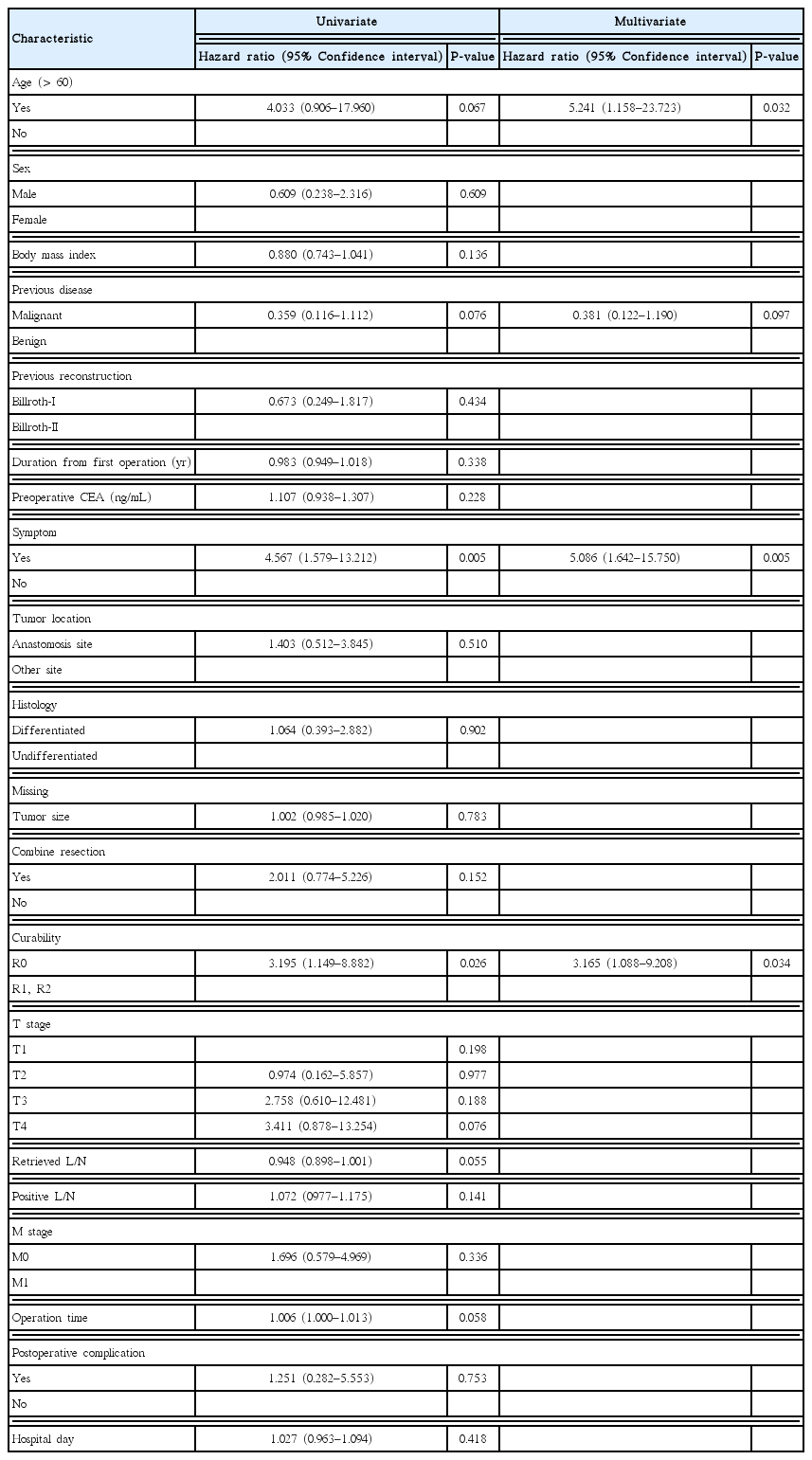
In the comparison of malignant disease (MD) and benign diseases (BD) group, the Billroth I:Billroth II ratio was 52.2% and 12.5%. The time interval from the previous operation to the diagnosis of remnant gastric cancer (RGC) was shorter in the MD group than in the BD group (6.6±6.04 vs. 34.7±10.12 years). Comparing B1 and B2 group, the proportion of patients previously undergone surgery due to MD was 85.7% and 44%. The time interval was higher in the B1 group than in the B2 group (8.0±8.78 vs. 23.8±16.48 years). Analyzing prognostic factors of survival, age and the presence of symptoms at the time of RGC diagnosis, and curability of surgery had a significant effect on the survival of the patients (P=0.032, hazard ratio [HR]=5.241, 95% confidence interval [CI], 1.158–23.723; P=0.005, HR=5.086, 95% CI, 1.642–15.750; P=0.034, HR=3.165, 95% CI, 1.088–9.208).
Conclusion
Patients who underwent partial gastrectomy for benign or MD require regular endoscopic follow-up and appropriate surgical approach is essential for the treatment of RGC.
INTRODUCTION
Remnant gastric cancer (RGC) was defined and studied initially as cancer developing in the remnant stomach after partial gastrectomy for peptic ulcer disease [1]. Although the number of patients undergoing gastrectomy for peptic ulcers has declined in recent years, due to the development of medical treatment [2], the incidence of cancer developing after partial gastrectomy for malignant disease (MD) has increased [3], becoming a clinical issue. This increase is attributable largely to the increased number of patients diagnosed with early gastric cancer, based on routine screening, and to the improved prognoses of patients with gastric cancer. Therefore, in addition to the original definition, RGC is now defined as gastric cancer developing in the remnant stomach, regardless of the reason for previous gastrectomy [4,5].
Because RGC occurs only rarely, due to its characteristic feature of development in the residual stomach, few cases have been reported. Even if it had been studied more extensively, the findings could not be applied to a large number of subjects [4,5]. Moreover, the characteristics and prognostic factors of gastric cancer studied to date are variable. In some reports, many cases of RGC were found in the advanced stage, and prognoses were poor relative to those for primary gastric cancer [6,7]. Other authors have reported no significant difference from primary gastric cancer [8,9]. Consequently, the clinicopathologic features of gastric cancer remain unclear, and few studies have examined the prognoses of affected patients.
In addition, due to the characteristics of RGC development, previous investigations have been conducted according to the reason for the previous operation [4,5] or the previous reconstruction method [10,11]. Few multifaceted reports on these studies, and no analysis of prognosis, have been published.
In this study, we divided patients diagnosed with RGC who underwent partial gastrectomy according to the reason for previous operation and the reconstruction method. We analyzed the clinical features, pathologic results, surgical outcomes, and survival rates to determine the clinical features and prognosis of RGC.
METHODS
Between January 2007 and February 2016, 2,106 patients underwent gastric cancer surgery at Inje University Busan Paik Hospital. Forty-one of these patients who had previously undergone partial gastrectomy were enrolled in this study. Two of these patients were excluded subsequently because they did not undergo gastrectomy due to peritoneal metastasis, resulting in a lack of pathologic information. Among thirty-nine patients, three patients were performed radical subtotal gastrectomy with Roux-en-Y anastomosis, and the other thirty-three patients underwent total gastrectomy. The Institutional Review Board of Busan Paik Hospital, Inje University, approved this study (IRB no. 16-0171).
The analysis was conducted in two ways. First, patients were divided into two groups according to the reason for previous partial gastrectomy: (1) MD and (2) benign diseases (BDs), such as peptic ulcer disease or abdominal injury. Second, the patients were divided into gastroduodenostomy (B1) and gastrojejunostomy (B2) groups. In B2 group, Braun anastomosis was not performed. We compared the clinicopathologic features and long-term outcomes between groups.
RGC was confirmed by preoperative endoscopic biopsy, and the tumor location was classified as anastomotic or non-anastomotic. The cancer stage was determined according to the International Union Against Cancer classification system, 7th edition [12]. Postoperative complications were classified according to the Clavien–Dindo grading system [13].
Statistical analysis was performed using SPSS software (ver. 23.0; SPSS Inc., Chicago, IL, USA). The independent-samples t-test and the Mann-Whitney test were used for continuous data, and the chi-squared test and Fisher’s exact test were used for categorical data. Survival curves were analyzed using the Kaplan-Meier method, and survival rates were compared between groups using the log-rank test. In all cases, P-values< 0.05 were considered to be significant.
Survival data through 28 February 2017 were acquired from the institution’s medical records. For patients lost to follow-up, survival and death data were obtained from the records of the National Statistical Office.
RESULTS
Clinicopathologic features of all patients
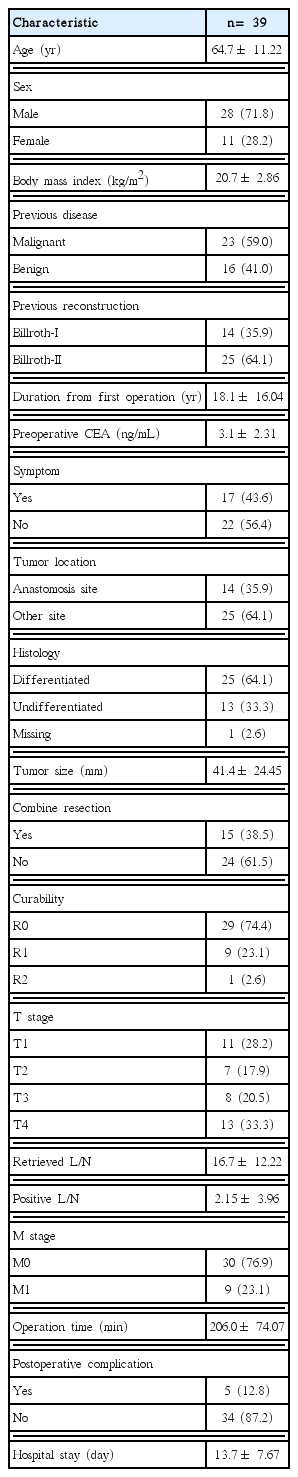
The mean age of the patients (28 [71.8%] males, 11 [28.2%] females) was 64.7 years. The mean body mass index (BMI) was 20.7 kg/m2. Twenty-three (59.0%) patients underwent previous surgery for MD and 16 (41.0%) patients underwent this surgery for BD. Fourteen (35.9%) surgeries involved gastroduodenostomy and 25 (64.1%) involved gastrojejunostomy; no other anastomotic procedure was performed.
The average time from the previous operation to the diagnosis of RGC was 18.1 years. 17 (43.6%) patients had symptoms such as weight loss, general weakness, and abdominal discomfort when they were diagnosed by endoscopy. On the other hand, 22 (56.4%) patients were diagnosed by endoscopy during gastric cancer follow-up program after surgery and health screenings. During operation, other organ resection except stomach was performed in 15 cases.
Pathologic R0 resection was possible in 29 (74.4%) patients; R1 and R2 resections were achieved in 9 (23.1%) patients and 1 (2.6%) patient, respectively. The stage was defined as T1 in 11 (28.2%) cases, T2 in 7 (17.9%) cases, T3 in 8 (20.5%) cases, and T4 in 13 (33.3%) cases. On average, 16.7± 12.22 lymph nodes were dissected.
Major (grade= 3) postoperative complications occurred in five (12.8%) patients. Grade 3 complications were peritoneal abscess in one case and pleural effusion in two cases. The grade 4 complication was postoperative bleeding, occurring in two cases. None of these patients died (Table 1).
Clinicopathologic characteristics according to disease at the time of first operation
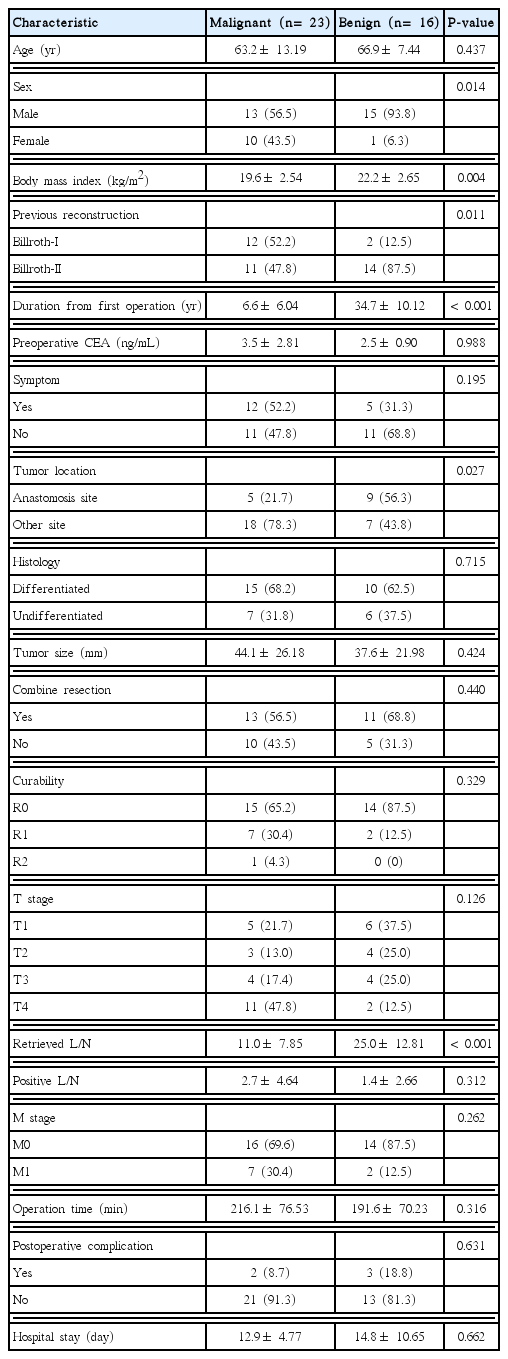
The proportion of male patients was smaller in the MD group than in the BD group (56.5% vs. 93.8%, P= 0.014). The BMI was also lower in the MD group than in the BD group (19.6± 2.54 vs. 22.2± 2.65 kg/m2, P= 0.004). In the previous reconstruction, the Billroth I:Billroth II ratio was 52.2% in the MD group and 12.5% in the BD group (P= 0.011). The time interval from the previous operation to the diagnosis of RGC was shorter in the MD group than in the BD group (6.6± 6.04 vs. 34.7± 10.12 years, P< 0.001).
The incidence of anastomotic site recurrence was lower in the MD group than in the BD group (21.7% vs. 56.3%, P= 0.027). The number of dissected lymph nodes was larger in the BD group than in the MD group (11.0± 7.85 vs. 25.0± 12.81, P< 0.001). No other significant difference between groups was observed (Table 2).
Clinicopathologic characteristics according to previous reconstruction method
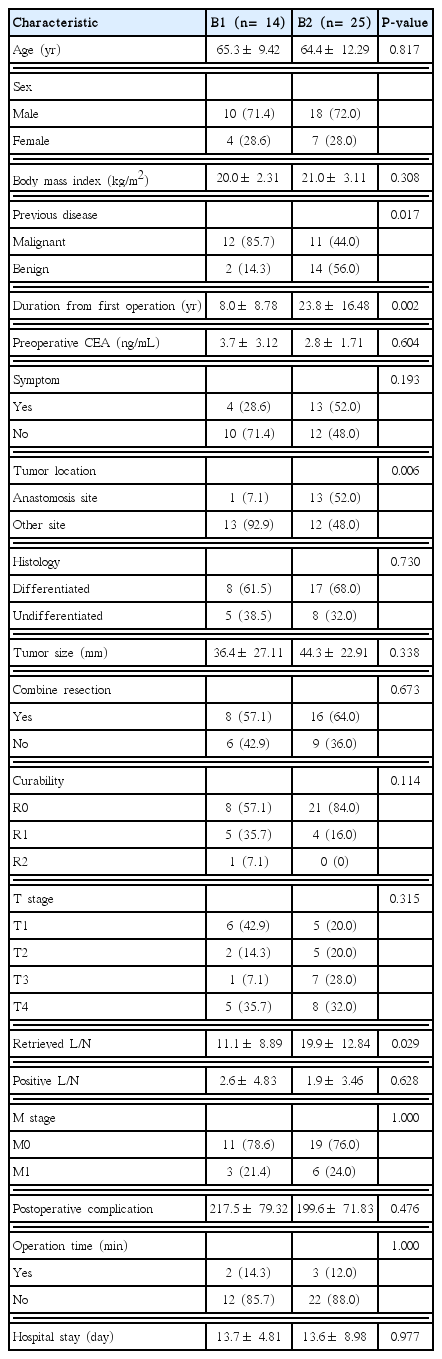
The proportion of patients who had previously undergone surgery due to MD was larger in the B1 group than in the B2 group (85.7% vs. 44%, P= 0.017). The time interval from the previous operation to the diagnosis of RGC was significantly higher in the B1 group than in the B2 group (8.0± 8.78 vs. 23.8± 16.48 years, P= 0.002).
The incidence of anastomotic site recurrence was lower in the B1 group than in the B2 group (7.1% vs. 52%, P= 0.006). The number of lymph nodes dissected was larger in the B2 group than in the B1 group (11.1± 8.89 vs. 19.9± 12.84, P= 0.029). No other significant difference between groups was observed (Table 3).
Survival
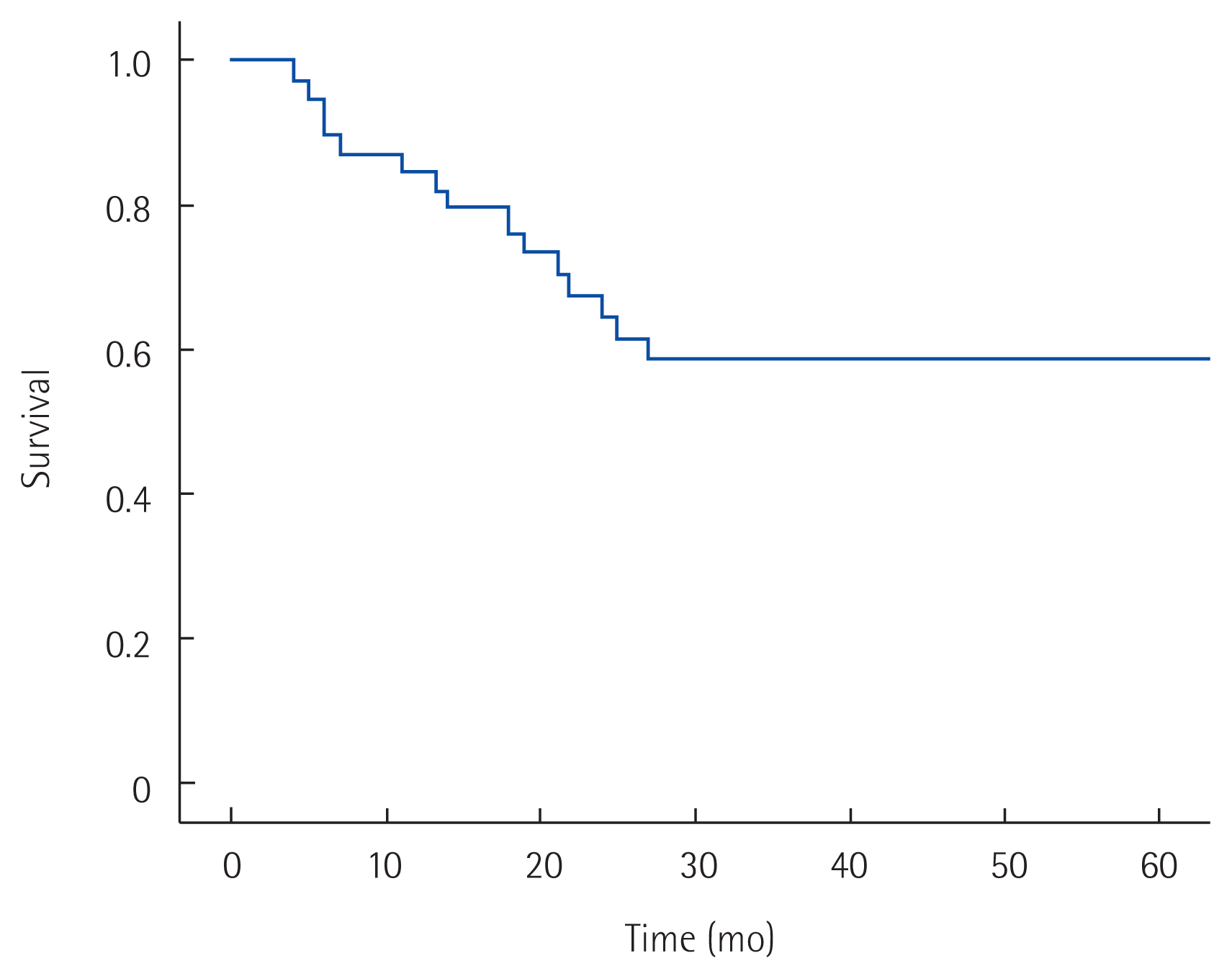
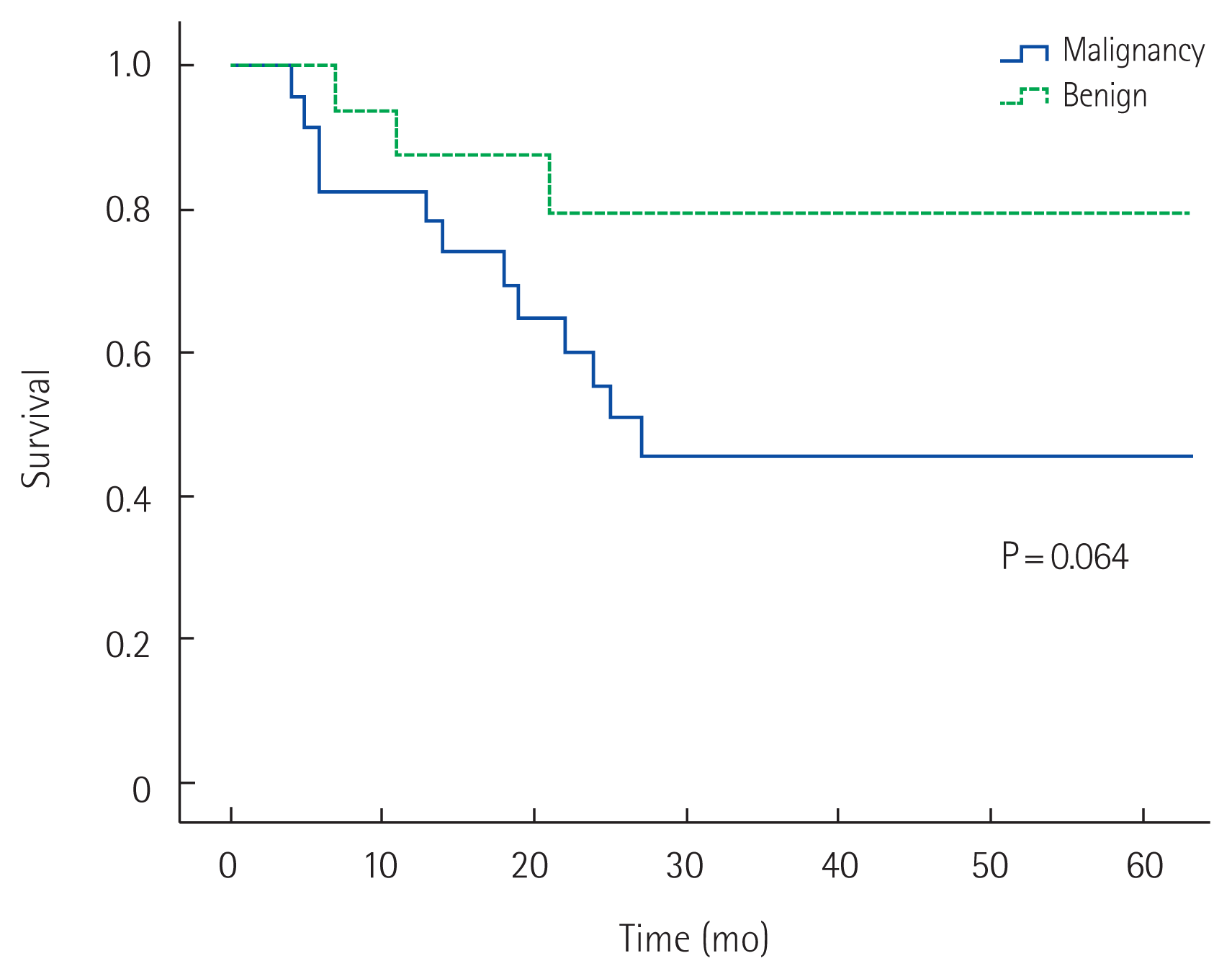
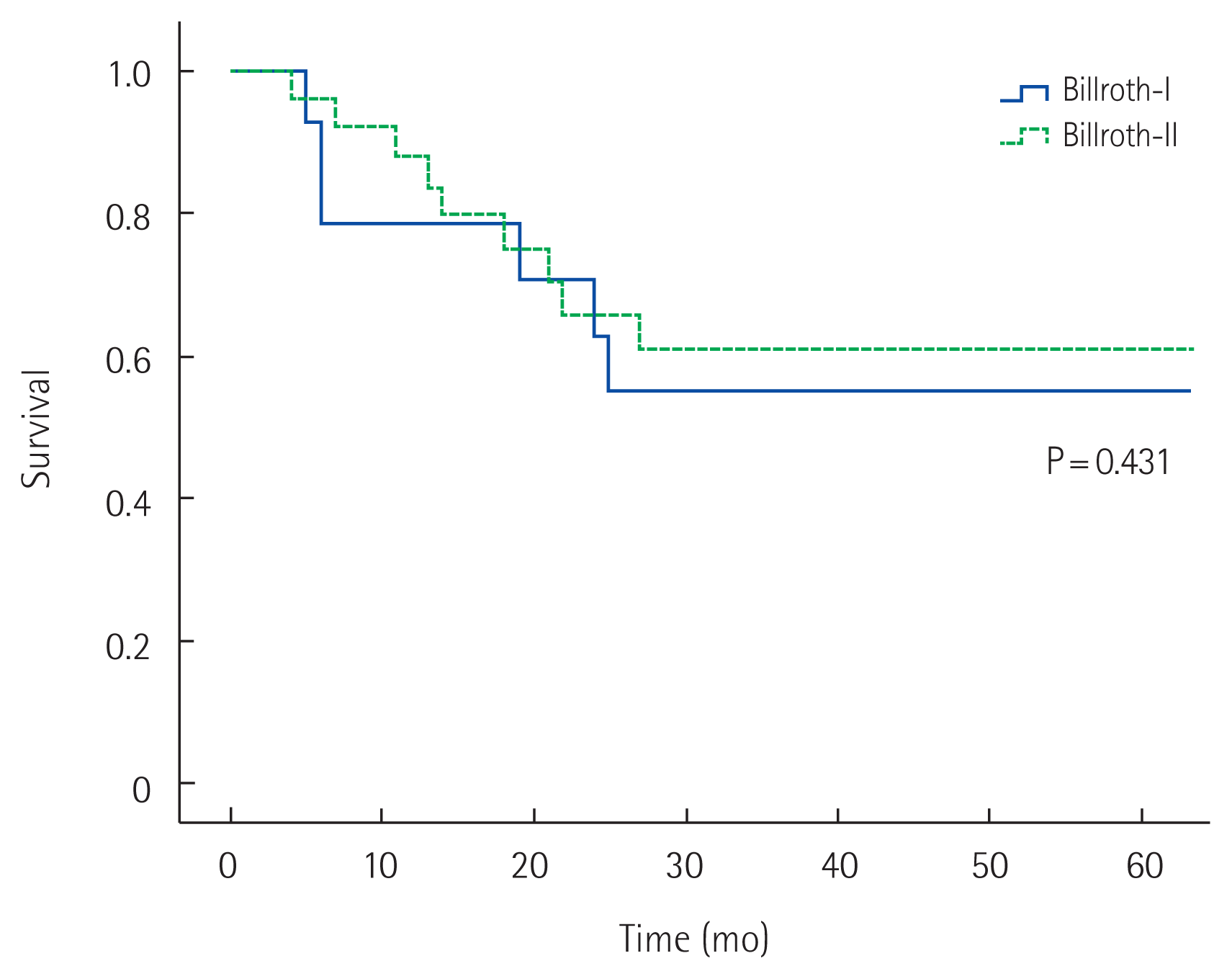
The 5-year overall survival rate of all patients was 58% (Fig. 1). The 5-year overall survival rates in the MD and BD groups were 45% and 79%, respectively; those in the B1 and B2 groups were 55% and 60%, respectively. No significant difference in survival was detected in either pair of groups (P= 0.064 and P= 0.431, respectively) (Figs. 2, 3). In univariate analysis, presence of symptoms and curability were significantly associated with overall survival (P=0.005, hazard ratio [HR]=4.567, 95% confidence interval [CI], 1.579–13.212; P= 0.026, HR= 3.195, 95% CI, 1.149–8.882). In multivariate analysis, age, presence of symptoms and curability was significantly associated with overall survival (P= 0.032, HR= 5.241, 95% CI, 1.158–23.723; P= 0.005, HR = 5.086, 95% CI, 1.642–15.750; P= 0.034, HR= 3.165, 95% CI, 1.088–9.208). In Fig. 4, there were significant differences in overall survival rates according to age, presence of symptoms and curability (P = 0.047, P = 0.019, P = 0.002) (Table 4).
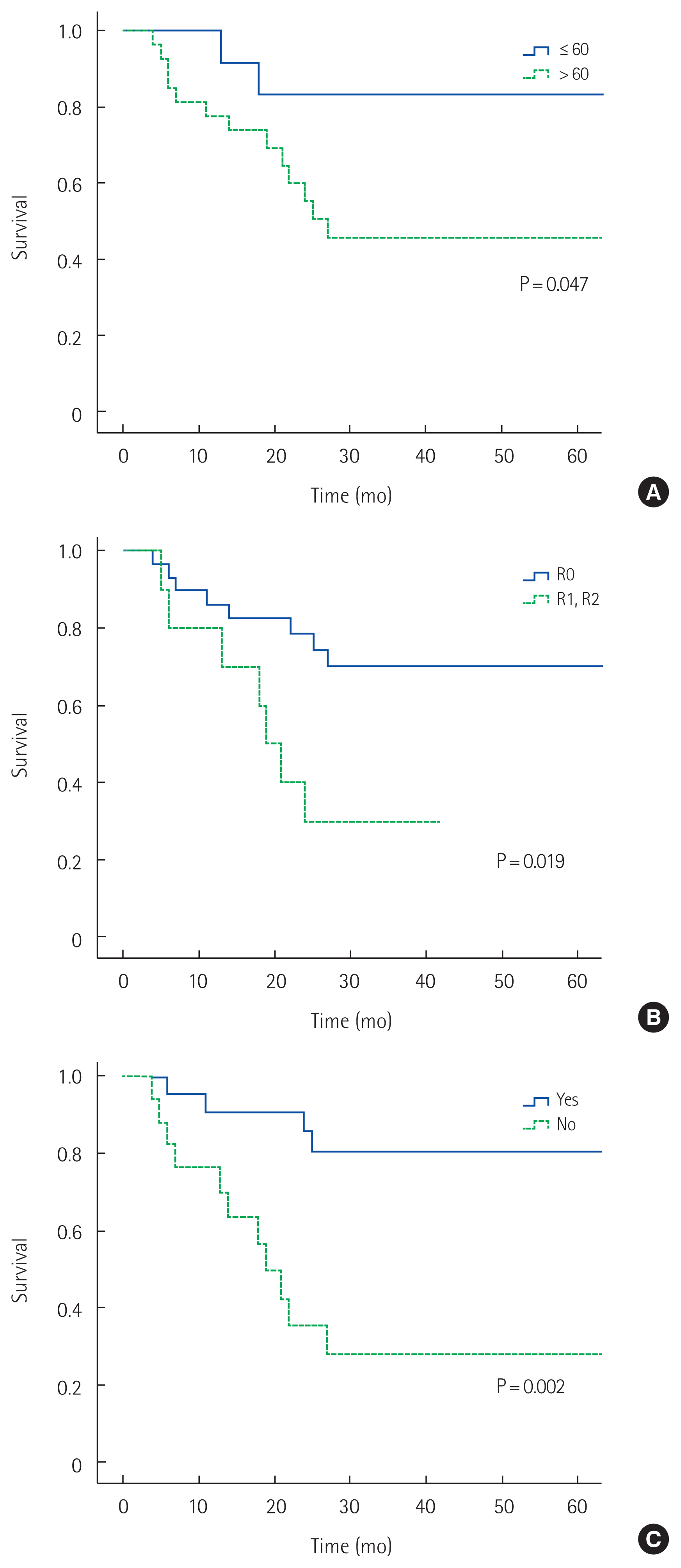
Overall survival rates according to prognostic factors. (A) Age, (B) Curability, and (C) Presence of symptoms. R0, no cancer cells seen microscopically at the resection margin; R1, cancer cells present microscopically at the resection margin; R2, macroscopic positive margin.
DISCUSSION
RGC was defined and studied initially as cancer developing in the remnant stomach after partial gastrectomy for peptic ulcer disease [1,14,15]. However, its definition has been changing. Recently, development of medical treatment has reduced the incidence of gastric resection due to benign disease such as peptic ulcer [2]. However, the increased detection of early gastric cancer [3] and increased survival rate of patients with gastric cancer have resulted in a continual increase in the number of patients diagnosed with RGC after partial gastrectomy for MD [4,5]. Moreover, RGC was initially defined as cancer occurring 5 or 10 years after previous operation [1,14,15]. However, recent reports have focused on this entity as cancer developing in the remnant stomach, regardless of the time since previous operation [4,5]. In this study, we also defined RGC as cancer developing in the remnant stomach, regardless of the reason for initial surgery or the time from previous operation to cancer development and studied its clinical features.
With regard to BD, the reflux of bile and pancreatic juice is considered to be the most important cancer-causing factor [16–18]. On the other hand, patient-specific pre-cancerous factors, such as already developed atrophic gastritis and intestinal metaplasia have more important effects on MD development [19,20]. Because these two developmental mechanisms may lead to differences in clinical characteristics, patients were divided into MD and BD groups for analysis in this study.
Comparing MD and BD groups, the difference in the interval from previous operation to diagnosis is caused by the reason mentioned above. The time interval from the previous operation to the diagnosis of RGC differed between the MD and BD groups (6.6 and 34.7 years, respectively), which is a previously reported characteristic of RGC. This disease has been reported to develop within 10 years of previous surgery in patients with MD, and within more than 20 years in those with BD [4,5,10].
The predominance of B2 reconstruction in the BD group is in agreement with previous reports; the relative frequencies of reconstruction methods used for MD differ slightly among reports [5, 10,11], possibly due to the tendency of surgeons to perform gastric surgery in such cases.
With regard to tumor site, anastomotic location was predominant in the BD group, whereas non-anastomotic location was predominant in the MD group. This difference reflects the higher ratio of B2:B1 reconstruction in the previous operation in the BD group and, in turn, leads to greater irritation of reflux at anastomosis sites, resulting in a high occurrence rate of the disease at this site, in those with BD. On the other hand, pre-cancerous factors affect the entire remnant stomach in patients with MD.
Some previous studies have documented that the risk of RGC development is 1.6 to 10 times higher in patients undergoing B2 reconstruction that in those undergoing B1 procedures, due to the reflux of bile and pancreatic juice [17,21–23]. Therefore, differences in clinical features were investigated according to reconstruction method in this study.
The differences between the B1 and B2 groups in the interval from previous operation to RGC diagnosis, and in the number of dissected lymph nodes, are due to the high ratio of previous operation due to MD in the B1 group and the high ratio of previous operation due to BD in the B2 group.
The incidence of anastomotic cancer was higher in the B2 group than in the B1 group because the reflux of bile and pancreatic juice contributed to cancer development at the anastomosis site in the B2 group, and because a larger proportion of patients in the B1 group underwent previous operations due to MD.
RGC is known to be difficult to treat and to be associated with a poor prognosis, as it is often diagnosed in the advanced stage and adhesion is severe (as determined intraoperatively) [6,9,24]. In our study, 23.1% of cases had distant metastasis, which is higher than the 10% reported for primary gastric surgery [25].
The incidence of postoperative complications was 12.8%, which is higher than the incidence of postoperative complications of primary gastric cancer surgery at our institution [26]. However, it is lower than the 20%–50% reported in previous studies [4,5,10], and similar to reports of a 10% incidence of complications after gastrectomy [27,28].
Grade 3 complications occurring in our sample were peritoneal abscess in one case and pleural effusion requiring percutaneous drainage in two cases. Grade 4 complications requiring treatment in the intensive care unit occurred in two cases. One patient underwent arterial embolization due to cystic artery bleeding, and the other patient required emergency operation under general anesthesia on the day of operation due to mesenteric arterial bleeding.
R0 resection was possible in 74.4% of cases, which is similar to the 60%–80% reported in previous studies. The 5-year overall survival rate was 50%, which was similar to the previously reported 40%–60% [14,24]. Because curability of surgery could affect the long term result of treatment, surgeons have to effort to complete resection of disease.
The most important diagnostic tool for the detection of RGC is endoscopy. Like primary gastric cancer, RGC is difficult to detect based on nonspecific symptoms, such as weight loss or abdominal discomfort. In Japan, endoscopic follow-up is recommended for up to 5 years after partial gastrectomy [29]. However, as the average period from the previous operation to the diagnosis of RGC is 6.6 years, periodic endoscopic examination is mandatory beyond this 5-year period. In addition, RGC may occur 30 or more years after gastric resection for BD. Therefore, endoscopic follow-up should not be neglected.
In this study, 43.6% of patients with RGC had symptoms. This finding is similar to previous reports of symptoms in 50%–70% of patients [5,10]. As presented in Fig. 4, the presence of symptoms in patients and age of patients are closely related to their long-term survival rate. Therefore, in patients who have previously undergone partial gastrectomy require regular endoscopy even if they have no symptoms.
We analyzed the clinical characteristics and prognoses of patients with RGC according to the reason for previous operation and the reconstruction method. Differences were observed between groups in the time from previous operation to RGC diagnosis and the tumor location, but not in the long-term survival rate. Radical resection was achieved in 74% of all patients, and the 5-year overall survival rate was 58%.
Given the characteristics of RGC, patients who have undergone partial gastrectomy require regular endoscopic follow-up. Upon the diagnosis of RGC, aggressive treatment, such as surgery aiming complete resection, is essential for long-term survival.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.