유방암 뇌전이 환자의 항암치료의 유용성
The effectiveness of chemotherapy in breast cancer patients with brain metastases
Article information
Abstract
Purpose:
Treatments of brain metastases in breast cancer include whole brain radiotherapy, surgery, stereotactic radiosurgery, and systemic chemotherapy. The aim of this study was to investigate the clinicopathological factors which were associated with brain metastases-related survival, and to evaluate the efficacy of systemic chemotherapy.
Methods:
A total of 106 breast cancer patients with brain metastases who were treated at the Seoul St. Mary’s Hospital were retrospectively analyzed. The brain metastases-free survival (BMFS) was defined as the time from first systemic metastases to detection of the brain metastases. Overall survival after brain metastases (OS) was measured from the detection of the brain metastases to death. The patient’s clinicopathological factors which were associated with BMFS and OS and role of systemic chemotherapy on brain metastases were evaluated.
Results:
The median BMFS was 30.7 months. In univariate analyses, age >50 years, stages I/II, tumor size <5 cm, positive lymph node ≤3, no vascular invasion, positive estrogen receptor, use of adjuvant chemotherapy, solitary brain lesion, and brain metastases as an initial recurrence site were associated with longer BMFS. The median OS after brain metastases was 5.0 months. In univariate analyses, nuclear grade I/II, mitotic activity indices <10, non-triple negative receptor, solitary brain lesion, and administration of local and systemic treatment on brain metastases were associated with longer OS. In multivariate analyses, systemic chemotherapy after brain metastases was the only significant prognostic factor associated with better OS and was effective regardless of blood-brain barrier (BBB) permeability.
Conclusion:
A systemic chemotherapy after brain metastases improved OS regardless of BBB permeability in breast cancer patients.
INTRODUCTION
Breast cancer is the second most common cause of brain metastases [1] and brain metastases are present in approximately 10%–16% of patients with metastatic breast cancer [2]. Diagnosed cases of brain metastases are increasing because of the improved detection of small metastases, using more advanced diagnostic methods. This has improved overall survival by introducing earlier and more effective systemic chemotherapies [3].
In most cases, breast cancer patients progressed to brain metastases after metastases had appeared systemically in the lung, liver, bone, and other organs [4]. Of the patients with brain metastases, 60%–70% had multiple brain lesions, and the median time from diagnosis of breast cancer to brain metastases was reported to be 35 months [5]. Brain metastases were the cause of death or a major contributing factor in 68% of the patients [6]. The median survival of untreated patients was as short as 1-2 months [7].
Several factors increased the risk of brain metastases, including young age, negative hormone receptors, large tumor sizes, positive lymph node status, previous lung, liver, or bone metastases, and increased numbers of metastatic sites [2,8-12]. In addition, recent studies have shown that the risk of brain metastases in overexpressed human epidermal growth factor receptor 2 (HER2) tumors increased [13]. However, no conclusions could be made as to whether there was a specific biological factor that caused a tumor to preferentially metastasize to the brain, or whether the brain metastases were related to a generalized worse metastatic potential.
Treatment of brain metastases consists of whole brain radiotherapy(WBRT), surgery, stereotactic radiosurgery (SRS), and systemic chemotherapy [2]. WBRT was considered a standard treatment for patients with brain metastases. The main purpose of WBRT was to relieve neurological symptoms caused by metastatic brain tumors. However, an additional study showed that younger patients with higher performance status scores, and controlled systemic disease, had longer median survivals after WBRT [14]. The goals of surgery are to obtain immediate symptom relief and to obtain localized control of the metastases. Neurosurgical resection of brain metastases,
in addition to WBRT, improved the median survival from 3 to 6months to 14 to 16 months [7,15]. However, surgery may be contraindicated for many patients because of comorbid conditions or surgically inaccessible tumor locations. SRS is another therapeutic modality, which is noninvasive and allows more than one lesion to be treated, including those in locations that are not surgically accessible. Systemic chemotherapy plays a limited role in the treatment of brain metastases. This is partially due to the unique structure of the blood-brain barrier (BBB). Several studies reported that it was more effective to use agents that crossed the BBB in the treatment of breast cancer with brain metastases [16].
The primary purpose of our study was to investigate the clinicopathological factors associated with brain metastases-free survival (BMFS) and overall survival after brain metastases in breast cancer patients. The second purpose was to investigate the efficacy of systemic chemotherapy, especially to determine whether BBB-crossing chemotherapeutic agents improved the overall survival rates.
METHODS
Among the patients diagnosed with breast cancer, between May 1986 and June 2007, at the Seoul St. Mary’s Hospital, the Catholic University of Korea, 168 patients with brain metastases were reviewed for this retrospective study. Among these patients, 106 patients with nearly complete medical records and pathological data were included in this study. Computed tomography or magnetic resonance imaging of the brain was used to diagnose brain metastases. Histological examination of the brain tumors was not performed for any patient. Age, tumor type, stage, proliferative index, estrogen receptor(ER) levels, progesterone receptor (PR) levels, HER2 status, local and systemic treatments, interval between first diagnosis of breast cancer and occurrence of brain metastases, and survival thereafter were analyzed from the patient database of our hospital. BMFS was defined as the time from first systemic metastases of breast cancer to detection of the brain metastases. Overall survival after brain metastases(OS) was measured from the time of detection of the brain metastases to the time of death. Univariate survival analysis was estimated using the Kaplan-Meier method and the log-rank test was used to assess survival differences between prognostic factors. Multivariate analysis was performed using the Cox proportional hazards regression model. A P-value less than 0.05 was considered statistically significant.
This study was approved by the institutional review committee of Seoul St. Mary’s Hospital (KC10RISI0358).
RESULTS
Patient characteristics
Table 1 shows the clinicopathologic data for the 106 breast cancer patients with brain metastases. The median age at diagnosis of brain metastases was 50 years (range, 27-73 years). The tumor stage showed 1/2/3/4 in 31 (31%)/48 (45%)/16 (15%)/9 (9%) patients and the nodal stage was 0/1/2/3 in 24 (23%)/27 (25%)/18 (17%)/37 (35%) patients, respectively. The initial tumor node metastasis (TNM) stage of patient was I, II, III, IV in 12 (11%)/35 (33%)/49 (46%)/10 (10%) in patients. Hormone receptor status of primary breast lesions demonstrated that 29 patients (38%) were ER positive, 48 patients (62%) were ER negative, 32 patients (42%) were PR positive, and 45 patients (58%) were PR negative. HER2 expression was analyzed by using immunohistochemistry or fluorescence in situ hybridization. Twenty-nine patients (41%) showed HER2 overexpression, and 41 patients (59%) were HER2 negative. Twenty-three (30%) patients had a triple receptor negative status. Among the proliferative markers, histological grade III was diagnosed in 45 cases (58%) and NG III was diagnosed in 35 cases (45%). Eleven (12%) patients had vascular invasion. The mitotic activity index (MAI), the number of mitoses in ten consecutive high power fields, was more than 10 in 44 (56%) patients.
Local and systemic treatment of brain metastases (Table 2). Brain metastases occurred as a single lesion in 27 (25%) patients and multiple lesions in 79 (75%) patients. The brain was the first metastatic site in 20 (19%) patients and 86 (81%) patients had extracranial metastases at brain metastases diagnosis. The most common metastatic sites at time of diagnosis of brain metastases were bone (60 patients) and lung (45 patients). After the diagnosis of brain metastases, WBRT was performed in 71 (67%) patients. Nine (9%) patients were treated with surgery or SRS and 12 patients (11%) were given combined treatment (WBRT and surgery/SRS). Fifty-five patients (52%) received systemic chemotherapy after the diagnosis of brain metastases. Twenty-one patients received BBB-crossing agents, such as capecitabine, methotrexate, carboplatin, cisplatin, and vinorelbine. Thirty-four patients received the BBB impermeable agents, such as docetaxel, paclitaxel, doxorubicin, and gemcitabine.
Survival analysis associated with clinicopathologic factors
Median BMFS was 30.7 months and median OS after brain metastases was 5.0 months. The BMFS and OS were analyzed according to the patient and tumor characteristics (Table 3). In univariate analyses, more than 50 years of age (P=0.002), stages I and II (P=0.024), tumor size less than 5 cm (P=0.026), lymph node number less than 3 (P=0.034), absence of vascular invasion (P=0.017), positive ER status (P=0.043), use of adjuvant chemotherapy (P=0.015), presence of a solitary metastatic brain lesion (P=0.024), and brain metastases as initial site of recurrence (P=0.001) were associated with longer BMFS.
Overall survival of patients with triple negative receptors was significantly shorter than for those with non-triple negative receptors (3.5 months vs. 5.4 months, P=0.011). Survival of patients with single metastases was significantly longer than for those with multiple metastases (11.1 months vs. 4.1 months, P=0.005). Patients who received combined local treatment (WBRT and SRS/surgery) had a longer survival than the patients who did not (17.4 months vs. 1.3 months, P=0.05). Survival of patients who received chemotherapy after brain metastases was significantly longer than for those who did not receive chemotherapy (10.1 months vs. 2.4 months, P<0.001). NG I and NG II (P=0.046), MAI less than 10 (P=0.008), non-triple receptor negative status (P=0.011), presence of solitary metastatic brain lesion (P=0.005), and if local (P=0.05) and systemic chemotherapeutic drug treatment was given (P<0.001) for brain metastases, were associated with longer OS (Fig. 1). With multivariate Cox regression analyses, most factors were not statistically associated with BMFS. However, systemic chemotherapy after brain metastases was a significant factor associated with better overall survival times (hazard ratio=8.091; 95% confidence interval, 3.700–17.697; P=0.000) (Table 4). Among patients that received systemic chemotherapy after brain metastases, patients were grouped into those given BBB-crossing agents and those given the impermeable agents. There were no statistically significant differences in clinicopathological features between the two confidence interval groups (data not shown), and the median OS were not statistically different between the two groups (15.6 months vs. 8.5 months, P=0.09) (Fig. 2).
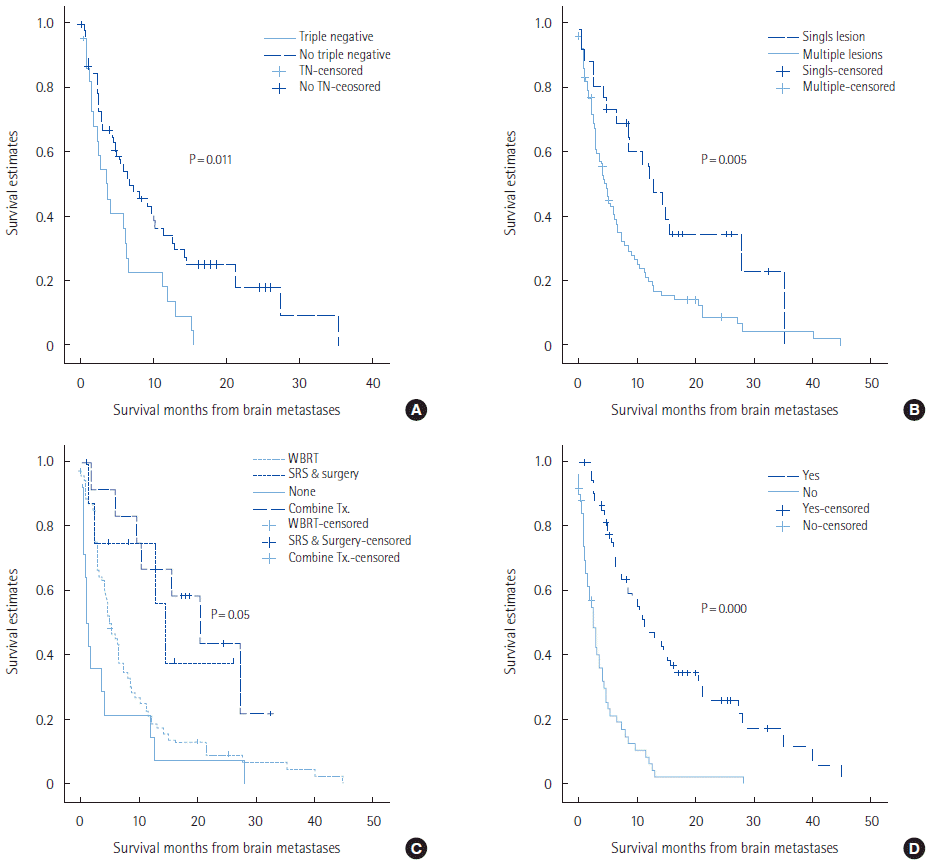
Kaplan-Meier overall survival curves according to (A) triple negative receptor status, (B) number of metastatic brain lesion, (C) brain metastases local treatment modalities, and (D) systemic chemotherapy after brain metastases. TN, triple negative; Tx, treatment; WBRT, whole brain radiation therapy; SRS, stereotactic radiosurgery.
DISCUSSION
In most reports, brain metastases occurred in the late stages of metastatic breast cancer and were already found in lung, liver, or bone by the time brain metastases was diagnosed. In breast cancer, the brain was rarely the first metastatic site [7]. The median time from diagnosis of breast cancer to brain metastases was reported to be 2 to 3 years, median survival time from the diagnosis of brain metastases was only 3 to 4 months, and a 1 year survival was reported in 10%–20% of the cases [6,7,17]. In our study, extracranial brain metastases was diagnosed in 86 (81%) of the patients and brain metastases as the initial site of recurrence was found in 20 (19%) patients. The time from first systemic metastases of breast cancer to detection of the brain metastases was 30.7 months, and overall median survival time was 4.95 months, with only 26% of the patients surviving for more than 1 year. Our results were similar to previous reports [7,8].
Tham et al. [8] reported a comparative analysis of risk factors in patients with central nervous system (CNS) metastases (n=383) versus in patients who had no CNS metastases (n=2,302). They found that younger age, ER negativity, low Bcl-2 levels, higher percent in S-phase, aneuploidy, and altered p53 were significantly associated with CNS metastases. However, tumor size, lymph node status, use of adjuvant systemic chemotherapy, and HER2 overexpression had little correlation with the risk of brain metastases. Gonzalez-Angulo et al. [10] showed that the characteristics significantly associated with more aggressive clinical presentation, including negative hormone receptor status, NG III classification, larger tumor size, positive lymph nodes, and a higher disease grade correlated with an ear-lier onset of brain metastases. Evans et al. [18] confirmed that patients under 50 years of age and with ER negative tumors, had a 53% risk of developing brain metastases. In our study, we found that certain features such as being under 50 years of age, having an advanced TNM stage, large tumor size, many positive lymph nodes, presence of vascular invasion, ER negativity, no use of adjuvant chemotherapy, multiple metastatic brain lesions, and the presence of extracranial metastases at brain metastases diagnosis, were associated with increased risk of brain metastases.
Local treatment modalities were associated with better outcomes. In previous studies, overall survival of patients with brain metastases, treated with supportive care alone, was 1 to 2 months, and WBRT improved the median survival to 3 to 6 months [19,20]. Surgical resection offered a further benefit of immediate decompression of the tumor mass effect, which improved the neurological symptoms and decreased the risk of brain herniation [19]. Overall, surgical resection improved the median survival time to 7 to 9 months. Patchell et al. [21] and Vecht et al. [22] randomized 48 and 63 patients, respectively, to surgical resection and WBRT, or WBRT alone. Both studies demonstrated a survival advantage for the combination approach over WBRT alone. However, Mintz et al. [23] conducted a similar study of 84 patients and found no survival benefit. SRS was less invasive than surgical resection and was the treatment of choice for patients who could not tolerate surgery or had surgically inaccessible lesions [19]. Furthermore, SRS also played a role in the management of recurrent brain metastases and could be administered safely to patients who had previously been treated with WBRT or surgical resection [24]. Overall, SRS extended the median survival to 7 to 13 months [25]. Kondziolka et al. [24] assessed 27 patients with multiple brain metastases treated with WBRT and SRS, or WBRT alone. Median time to failure was 36 months in patients treated with the combination approach versus 6 months in patients who received WBRT alone. The patients treated with WBRT and SRS had a median survival of 11 months vs. 7.5 months for the WBRT-alone treated patients; however, the survival difference was not significant. In our study, the median overall survival time was 4.8 months for patients who received WBRT, 8.3 months for those who received SRS/surgery, 17.4 months for those who received multiple treatment (WBRT and SRS/surgery), and 1.3 months for those who received no treatment. Our study demonstrated survival benefits for the multiple local treatment group over the WBRT alone or only SRS/surgery groups (P=0.05).
The role of systemic chemotherapy in patients with brain metastases is still unclear. Many patients with brain metastases have been heavily pretreated with chemotherapy directed at their systemic metastatic disease. Development of brain metastases is a later event in the natural history of most tumors, and this could reflect the failure of several prior chemotherapeutic regimens, and would suggest that the recurrent tumor histology is inherently more drug resistant [26]. An intact BBB limits the entry of most chemotherapeutic agents into the CNS. Treatment efficacy was determined by the sensitivity of tumor cells to chemotherapeutic agents, and whether or not these drugs could cross the BBB. In general, the chemotherapeutic drugs, such as docetaxel, paclitaxel, doxorubicin, and gemcitabine, do not cross the BBB. However, methotrexate, cisplatin, carboplatin, vinorelbine, topotecan, and capecitabine could cross the BBB [26]. High dose methotrexate achieved high concentrations in the CNS, obtaining 22% partial response, and 35% disease stabilization [27]. Cocconi et al. [28], reported a 55% response and a 13 months median survival time in patients treated with cisplatin and etoposide. Wang et al. [29] demonstrated a prolonged partial response to capecitabine, which was also demonstrated in patients previously treated with 5-fluorouracil, paclitaxel, tamoxifen, procarbazine, lomustine, and thalidomide. In our study, 55 patients (52%) received systemic chemotherapy after the diagnosis of brain metastases. Twenty-one patients received BBB crossing agents such as capecitabine, methotrexate, carboplatin, cisplatin, and vinorelbine. Thirty-four patients received BBB impermeable agents such as docetaxel, paclitaxel, doxorubicin, and gemcitabine. The median survival time of the patients who used the BBB permeable agents was longer than patients who used the agents which did not cross the BBB (15.6 months vs. 8.5 months), but there was no significance between the two groups (P=0.09). In a preclinical study, Fidler et al. [30] demonstrated that, while the BBB was intact in patients with the presence of small metastases, it was disrupted in those with large tumors. We could say that our results might support their suggestion.
Our study had several limitations. It was a retrospective design, had no control group, and responses to systemic chemotherapy were determined from the medical charts rather than by a rigorous evaluation of radiologic studies and protocol response criteria. In some cases, patient data were not complete. Another limitation was the small number of patients. As in other studies, treatment of breast cancer consisted of multimodality treatments, including hormonal therapy and radiotherapy. Most metastatic breast cancer patients received many chemotherapeutic agents before brain metastases were diagnosed. These variables may have an influence as confounding factors on overall survival.
This study did not analyzed other significant prognostic factors, such as performance status at brain metastasis diagnosis, the first systemic metastasis site before brain metastasis, and treatment after the first systemic metastasis.
So, even though systemic chemotherapy after brain metastases was a significant factor associated with better overall survival times in multivariate analysis, we should interpret the results with care.
Nevertheless, in contrast to previous studies, we classified patients who were given the BBB-crossing agents and the non-crossing agents. In this regard, our study is significant, because we found that there was no survival benefit to the patients who received the agents that crossed the BBB.
In this study, we identified the clinicopathological factors associated the overall survival rate after brain metastases in breast cancer patients. Among these factors, systemic chemotherapy after brain metastases was identified as the most important factor. Although survival of breast cancer patients with brain metastases was generally poor, overall survival seemed to be affected by aggressive systemic chemotherapy after brain metastases. Our results differ from previous studies, which found a survival benefit only from agents which were able to cross the BBB. Therefore, it is important to use an agent known to be active against breast cancer regardless of whether it crosses the BBB. In the future, extensive prospective randomized trials are still needed to fully assess the efficacy of BBB-crossing and non-crossing chemotherapeutic agents in breast cancer with brain metastases.
Notes
No potential conflict of interest relevant to this article was reported.