ABSTRACTPurposeThe present study compares the peri/postoperative and oncological outcomes of abdominoperineal resections (APR) and sphincter saving resection (SSR) for low lying rectal cancer.
MethodsBetween January 2001 and December 2014, 176 patients who underwent SSR (n=67) and APR (n=109) for low rectal cancer, without stage IV, were retrieved from a retrospective database.
ResultsWith a median follow-up of 66.5 months. The mean total number of harvested lymph nodes was 16.7 (SSR) versus 17.1 (APR) (P=0.801). The advanced T stage was higher in the APR group (82.6%) versus the SSR group (55.2%) (P=0.006). The positive rate of lymph nodes after surgery was significantly higher in the APR group (45.9%) versus SSR group (25.4%) (P<0.05). The 5-year overall survival rates for SSR and APR were 87.3% and 67.6%, respectively (P<0.005). The 5-year disease-free survival rate (DFS) was 83.6% (SSR) versus 65.5% (APR) (P=0.002). The recurrence rate was higher in the APR group (34.9%) versus the SSR group (14.9%) (P=0.004). Local recurrence rate was not different between the two groups. However, distant recurrence rate was significantly higher in the APR group (26.6% vs. 11.9%, P=0.023). In multivariate analysis, node positive (N0 vs. N1–2) was an independent prognostic factor for DFS (P<0.005).
INTRODUCTIONThe standard surgery for low lying rectal cancer is abdominoperineal resection (APR) which requires a permanent colostomy. However, ultra-low anterior resection (uLAR) with coloanal anastomosis (CAA) and intersphincteric resection (ISR) with CAA have recently been performed for low lying rectal cancer as an anus preserving alternative that avoids the need for permanent colostomy [1,2]. Sphincter saving surgery (SSR) such as uLAR and ISR has recently increased with the better understanding of anal sphincter anatomy, enhanced surgical techniques, and neoadjuvant chemoradiation therapy (CRTx) [3]. Neoadjuvant CRTx contributes to enhancing the success rates of SSR (uLAR and ISR with CAA) by downstaging and downsizing the tumor [4,5]. Although SSR (uLAR and ISR with CAA) has recently been conducted more frequently, this surgery method can increase the potential recurrence risk. Preserving the anal canal and levator ani muscles may potentially damage the distal resection margin (DRM) and circumferential resection margin (CRM) [6–8]. Compared to SSR, APR is considered to enable a longer and more reliable DRM and CRM. It can also resect and obtain more tissue, including the anal canal and the surrounding tissue. Thus, APR is regarded to have a more favorable oncological outcome than that SSR. Nevertheless, in previous studies comparing SSR and APR, several studies reported that the local recurrence and/or 5-year survival rate are worse in APR than SSR for low rectal cancer [9,10]. The aim of the present study is to compare the peri/postoperative and oncological outcomes of APR and SSR for low rectal cancer.
METHODSBetween January 2001 and December 2014, 176 patients that underwent curative surgical resection (uLAR, ISR, and APR) for low rectal adenocarcinoma (≤5 cm from anal verge) were identified from a retrospective database. Stage IV patients in the present study were excluded. Among the study participants, 67 patients underwent SSR (uLAR and ISR with CAA) and 109 patients underwent APR. All data of the clinical and pathological features were reviewed retrospectively. All patients underwent colonoscopy and biopsy, staging scans (computed tomography scan chest, abdomen and pelvis/magnetic resonance imaging pelvis) and occasionally positron emission tomography-computed tomography scans. The neoadjuvant CRTx was performed with long course CRTx (5-fluorouracil based chemotherapy, 50.4 Gy) as the patients were clinically T3 or T4 and/or node positive. Repeat scans were performed after a minimum of 4 weeks of CRTx completion. Surgery was performed 6 to 8 weeks after completion of CRTx. All patients received full bowel preparation and a single shot of prophylactic antibiotics. All patients underwent complete total mesorectal excision. Adjuvant chemotherapy was performed on a 5-fluorouracil and leucovorin-based regimen (six cycles of monthly bolus intravenous 5-fluorouracil [400–425 mg/m2/day], days 1–5; and leucovorin [20 mg/m2/day], days 1–5). The adjuvant chemotherapy was not performed in the cases of old age or patient refusal or side effects. Patients received close follow-up and were recorded on a database till death or May 2018. Disease-free survival (DFS) was defined from the date of surgery to the date of the detection of recurrence or last follow-up or death. Patients in the two groups undergoing SSR (uLAR and ISR with CAA) and APR were compared with respect to demographics and oncological outcomes.
All statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA) and SPSS software, version 24.0 (IBM Corp., Armonk, NY, USA). Categorical variables were analyzed using the chi-square or Fisher exact test, and continuous variables were analyzed using the Student t-test and Mann-Whitney U rank test. Cumulative-incidence methods were used to estimate the rate of cancer recurrence. Overall survival (OS) and DFS were analyzed using the Kaplan-Meier method, and comparison was performed using the log-rank test. P-values of less than 0.05 were considered statistically significant.
RESULTSPatient characteristicsComparative analysis was conducted regarding the characteristics of the patients of the SSR group (n=67) and APR group (n=109) (Table 1). Mean age, sex ratio, height, weight, body mass index (BMI), and American Society of Anesthesiologists scores, as well as initial carcinoembryonic antigen level, were not significantly different between the two groups. The rate of patients who received neoadjuvant CRTx was significantly higher in the SSR group (52.2%) than in the APR group (14.7%) (P<0.005).
Pathologic resultsThe tumor-node-metastasis (TNM) stage, pT stage, pN stage, and pM stage were classified according to the American Joint Committee on Cancer (AJCC, 7th edition) staging system. Regarding the TNM stage distribution, the T stage was significantly different between the two groups (P<0.006) with more advanced stage in the APR group (T3 stage; 52.2% vs. 73.4%). As to the overall stage, the APR group showed a significantly higher proportion of advanced stage compared to the SSR group (IIIb; 13.4% in SSR vs. 34.9% in APR, P<0.003). There was no significant difference between the two groups regarding N stage (P=0.071) (Table 2). However, the rate of N (+) was higher in the APR group (n=50, 45.9%) than in the SSR group (n=17, 25.4%) (P<0.05). The histological grades of differentiation, perineural invasion rate and harvested number of lymph node were not significantly different between the two groups. The lymphovascular invasion rate was significantly higher in the APR group compared to the SSR group (APR 32.1% vs. SSR 16.4%, P=0.021). The proximal resection margin (APR 20.7 cm vs. SSR 14.1 cm, P=0.001) and DRM (APR 3.7 cm vs. SSR 2.1 cm, P=0.008) were longer in the APR group than the SSR group. Mass size and CRM were not significantly different between the two groups (Table 2).
Oncological outcomesThe mean follow-up period was 66.5 months. The 5-year OS rate was significantly higher in the SSR group compared to the APR group (87.3% vs. 67.6%, P<0.005). The 5-year DFS rate was also significantly higher in the SSR group than the APR group (83.6% vs. 65.5%, P<0.002) (Fig. 1). In the node negative stage, the 5-year OS rate (SSR 92.3% vs. APR 87.1%, P=0.100) and DFS rate (SSR 88.2% vs. APR 87.3%, P=0.488) were not significantly different between the two groups. In the node positive stage, the 5-year OS rate (SSR 74.2% vs. APR 45.2%, P<0.005) and DFS rate (SSR 70.6% vs. APR 39.6%, P<0.005) were significantly higher in the SSR group (Fig. 2). Regarding the oncological outcomes of neoadjuvant CRTx, patients that received neoadjuvant CRTx compared to those that did not showed significantly better results. The 5-year OS rate was 90.9% in patients that received neoadjuvant CRTx and 68.7% in those that did not receive neoadjuvant CRTx (P=0.001). The 5-year DFS rate was 83.0% in patients that underwent neoadjuvant CRTx treatment and 68.1% in those that did not (P=0.026) (Fig. 3).
Recurrent patterns of SSR versus APR for low rectal cancerThe total recurrence rate was significantly higher in the APR group compared to the SSR group (34.9% vs. 14.9%, P=0.004). The APR group also showed a significantly higher systemic recurrence rate (26.6% vs. 11.9%, P=0.023). The local recurrence rate was not significantly different between the two groups (9.2% vs. 3.0%, P=0.114). The organs of systemic recurrence were the lung, liver, para-aortic node, spine and inguinal node. The recurrence of the lung was observed in six patients (9.0%) of the SSR group and in 13 patients (11.9%) of the APR group. Liver recurrence was observed in two patients (3.0%) of the SSR group and in seven patients (6.4%) of the APR group. The recurrence of the para-aortic node was observed in three patients (2.8%) of the APR group. Spinal recurrence was observed in one patient (1.5%) of the SSR group and in three patients (2.8%) of the APR group. The recurrence of the inguinal node was observed in two patients (1.8%) of the APR group. The sites of local recurrence included the pelvic side wall, perineum, rectal fossa, and anastomosis site. The recurrence of the pelvic side wall was found in one patient (1.5%) of the SSR group and in two patients (1.8%) of the APR group. The recurrence of the perineum was in three patients (2.8%) of the APR group. The recurrence of the rectal fossa was in five patients (4.6%) of the APR group. The recurrence of anastomosis site was in one patient of the SSR group (1.5%) (Table 3).
Positive rate of pathologic lymph nodes associated with neoadjuvant CRTxThe node positive rate after surgery was significantly higher in patients that did not receive neoadjuvant CRTx (n=54, 43.2%) compared to those that did undergo the treatment (n=13, 25.5%) (P=0.020). The node negative rate after surgery was 74.5% (n=38) in patients that received neoadjuvant CRTx, and 56.8% (n=71) in patients that did not receive the treatment. The rate of patients who received neoadjuvant CRTx in the SSR group (n=35, 52.2%) was significantly higher compared to the APR group (n=16, 14.7%) (P<0.005). The node positive rate after surgery was significantly higher in the APR group (n=50, 45.9%) compared to the SSR group (n=17, 25.4%) (P<0.05) (Table 4).
Univariate and multivariate analyses for prognostic factorIn univariate analysis, BMI, tumor size, advanced T stage, N (+) stage, histologic grade, lymphovascular invasion, perineural invasion, and neoadjuvant CRTx treatment were prognostic factors of DFS after surgery for low lying rectal cancer. In multivariate analysis, BMI and N (+) stage were significant prognostic factors of DFS (Table 5).
DISCUSSIONDecisions regarding the appropriate surgical method for low lying rectal cancer are difficult to make. Oncologic surgery was designed for the purpose of radical resection, but recent oncologic surgery strives to preserve organs as much as possible. Regarding surgical procedures, APR and SSR share similar total mesorectal excision (TME) procedures which include the mesorectum tapering into the plane between the levator-sphincter junctions and intersphincteric plane. However, differences arise during the perineal procedure of APR during which the anal canal and the surrounding tissue may be obtained. Therefore, APR is expected to be more advantageous in terms of oncological outcomes after surgery by obtaining a longer DRM and a more secure CRM.
However, in the present study, the SSR group showed better oncological outcomes than the APR group. DRM was longer in the APR group compared to the SSR group (APR 3.7 cm vs. SSR 2.1 cm, P=0.008). The type of surgery for lower rectal cancer is largely determined by R0 resection and tumor location with adequate DRM (usually 2 cm allowed) [11]. However, this concept of a 2 cm DRM for lower rectal cancer has been challenged by many studies in the setting of neoadjuvant therapy. Study cases of locally advanced rectal cancer after receiving neoadjuvant CRTx reported that 21% of patients had distal margins less than 1 cm without a positive distal margin, and the extent of the DRM was not associated with DFS [12,13]. In the present study, although the APR group showed longer distal margin than the SSR group, this does not seem to have an effect on the oncological outcome.
Some studies have shown that APR is associated with increased local recurrence and decreased cancer-specific survival rate compared to LAR [9,14]. A propensity score matched SEER analysis reported worse oncological outcomes in patients with APR compared to CAA. The 5-year OS rate was 65.6% in APR versus 76.7% in CAA (P<0.001) [15]. The GRECCAR 1 study reported that OS rate and DFS rate were significantly higher for SSR (72.2% and 60.1%) compared APR (54.7% and 38.3%, respectively) [16].
In the present study, 5-year OS rate and DFS rate of APR (OS 67.6%, DFS 65.5%) were significantly worse compared to SSR (OS 87.3%, DFS 83.6%). The local recurrence rate was not significantly different between the APR and SSR groups (APR 9.2% vs. SSR 3.0%, P=0.114). However, the systemic recurrence rate was significantly higher in the APR group (APR 26.6% vs. SSR 11.9%, P=0.023). In another study similar to the present study, there was no difference in local recurrence but systemic recurrence was reported higher for APR than SSR (APR 29.1% vs. SSR 6.1%) [17]. Other studies reported that APR itself was not associated with increased local recurrence or reduced OS [18].
Regarding this study, the lymphovascular invasion rate was also significantly higher in APR than SSR (APR 32.1% vs. SSR 16.4%, P=0.021). The most common site of systemic recurrence was lung metastasis in both groups (APR 11.9%, SSR 9.0%). It is known that lower rectal cancer has dual venous drainage channels with portal and systemic communications through the superior rectal and internal iliac vein, respectively [19]. The lung is the most common site of extra-abdominal metastases in patients with colorectal cancer, and it occurs more often in patients with rectal cancer than colon cancer [20]. With higher lymphovascular invasion rates, higher systemic recurrence rates may be expected.
The CRM positive rate after surgery for rectal cancer was an important factor of recurrence and oncological outcome in a previous meta-analysis study [21]. In the present study, CRM positive rate was not significantly different between the APR and SSR groups (APR 4.6% vs. SSR 1.5%, P=0.252). Although it is more convenient to obtain CRM through APR, it is not difficult to obtain CRM with SSR. Moreover, the neoadjuvant CRTx may further reduce differences regarding the CRM positive rate. It seems that the difference in surgical type (APR or SSR) does not significantly affect CRM in the present study.
In another study of lower rectal cancer patients treated by neoadjuvant CRTx and TME, the 5-year release-free survival rates for the SSR, ISR, and APR groups were reported as 85%, 83%, and 47%, respectively (P=0.001) [22]. This study suggested the relatively poor oncological outcome of the APR group was due to the fact that patients with good response for neoadjuvant CRTx received ISR and those with poor response received APR. Neoadjuvant CRTx is effective in downstaging, increasing resection rate and local control rate, especially for patients with low rectal cancer, and thus increases the OS rate for certain patients [4,23,24]. Neoadjuvant CRTx can downstage tumors, which is illustrated with the decreased thickness of invaded intestinal wall and decreased numbers of metastatic lymph nodes. Lymph node metastasis (or N stage) is a significant prognostic factor for local recurrence, distant metastasis and OS rate [25–27].
In the present study, the rate of patients who received neoadjuvant CRTx in the SSR group was significantly higher compared to the APR group (SSR 52.2% vs. APR 14.7%) (P<0.005). The lower neoadjuvant CRTx rate in the APR group is because sphincter preservation is not necessary in a situation where en-bloc resection is judged to be possible in clinical stage. Due to this reason, surgery is first performed without neoadjuvant CRTx and then adjuvant chemotherapy is performed according to the pathologic stage. The lymph node (or N stage) positive rate after surgery was significantly higher in patients who did not receive neoadjuvant CRTx (n=54, 43.2%) compared to patients who received neoadjuvant CRTx (n=13, 25.5%) (P=0.020). When the patients were divided into groups according to CRTx treatment, the oncological outcome was significantly better in the CRTx treatment group. It is estimated that the oncological outcome of the APR group was worse than that of the SSR group because the lymph node positive (or N stage) rate was higher and the CRTx treatment rate was lower than the SSR group. The present study has several limitations. It is a retrospective, single-center study with small sample size and has significant selection biases.
In conclusion, the 5-year oncological outcome of the SSR group showed better results than the APR group. The higher positive rate of lymph nodes in N stage after surgery and the lower rate of neoadjuvant CRTx seem to have an effect on the oncological outcomes of the APR group.
REFERENCES1. Schiessel R, Karner-Hanusch J, Herbst F, Teleky B, Wunderlich M. Intersphincteric resection for low rectal tumours. Br J Surg 1994;81:1376-8.
2. Rullier E, Laurent C, Bretagnol F, Rullier A, Vendrely V, Zerbib F. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg 2005;241:465-9.
3. Baek SJ, Al-Asari S, Jeong DH, Hur H, Min BS, Baik SH, et al. Robotic versus laparoscopic coloanal anastomosis with or without intersphincteric resection for rectal cancer. Surg Endosc 2013;27:4157-63.
4. Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30.
5. Chetty R, McCarthy AJ. Neoadjuvant chemoradiation and rectal cancer. J Clin Pathol 2019;72:97-101.
6. Bannon JP, Marks GJ, Mohiuddin M, Rakinic J, Jian NZ, Nagle D. Radical and local excisional methods of sphincter-sparing surgery after high-dose radiation for cancer of the distal 3 cm of the rectum. Ann Surg Oncol 1995;2:221-7.
7. Saito N, Moriya Y, Shirouzu K, Maeda K, Mochizuki H, Koda K, et al. Intersphincteric resection in patients with very low rectal cancer: a review of the Japanese experience. Dis Colon Rectum 2006;49(10 Suppl):S13-22.
8. Koyama M, Murata A, Sakamoto Y, Morohashi H, Takahashi S, Yoshida E, et al. Long-term clinical and functional results of intersphincteric resection for lower rectal cancer. Ann Surg Oncol 2014;21:Suppl 3. S422-8.
9. Marr R, Birbeck K, Garvican J, Macklin CP, Tiffin NJ, Parsons WJ, et al. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg 2005;242:74-82.
10. Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Soreide O, et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum 2004;47:48-58.
11. Park IJ, Kim JC. Adequate length of the distal resection margin in rectal cancer: from the oncological point of view. J Gastrointest Surg 2010;14:1331-7.
12. Kuvshinoff B, Maghfoor I, Miedema B, Bryer M, Westgate S, Wilkes J, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: are < or = 1 cm distal margins sufficient? Ann Surg Oncol 2001;8:163-9.
13. Mezhir JJ, Shia J, Riedel E, Temple LK, Nash GM, Weiser MR, et al. Whole-mount pathologic analysis of rectal cancer following neoadjuvant therapy: implications of margin status on long-term oncologic outcome. Ann Surg 2012;256:274-9.
14. Perry WB, Connaughton JC. Abdominoperineal resection: how is it done and what are the results? Clin Colon Rectal Surg 2007;20:213-20.
15. Warschkow R, Ebinger SM, Brunner W, Schmied BM, Marti L. Survival after abdominoperineal and sphincter-preserving resection in nonmetastatic rectal cancer: a population-based time-trend and Propensity Score-matched SEER analysis. Gastroenterol Res Pract 2017;2017:6058907.
16. Rouanet P, Rivoire M, Gourgou S, Lelong B, Rullier E, Jafari M, et al. Sphincter-saving surgery for ultra-low rectal carcinoma initially indicated for abdominoperineal resection: Is it safe on a long-term follow-up? J Surg Oncol 2021;123:299-310.
17. Yeom SS, Park IJ, Jung SW, Oh SH, Lee JL, Yoon YS, et al. Outcomes of patients with abdominoperineal resection (APR) and low anterior resection (LAR) who had very low rectal cancer. Medicine (Baltimore) 2017;96:e8249.
18. Kim JC, Yu CS, Lim SB, Kim CW, Kim JH, Kim TW. Abdominoperineal resection and low anterior resection: comparison of long-term oncologic outcome in matched patients with lower rectal cancer. Int J Colorectal Dis 2013;28:493-501.
19. Warwick R, Williams PL. Angiology. Gray H, Warwick R, Williams PL, editors. Gray’s anatomy. Philadelphia, PA: Saunders; 1973. p. 706-9.
20. Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut 2010;59:1383-8.
21. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008;26:303-12.
22. Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg 2009;249:236-42.
23. Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Pahlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008;26:3687-94.
24. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23.
25. Rodel C, Grabenbauer GG, Papadopoulos T, Bigalke M, Gunther K, Schick C, et al. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys 2002;52:294-303.
Fig. 1Oncologic outcome between sphincter saving surgery (SSR) and abdominoperineal resection (APR) groups. (A) Overall survival rate and (B) disease-free survival rate. 
Fig. 2Oncologic outcome between sphincter saving surgery (SSR) and abdominoperineal resection (APR) groups according to N stage. (A, B) Five-year overall survival rate and disease-free survival rate for N (−) stage. (C, D) Five-year overall survival rate and disease-free survival rate for N (+) stage. 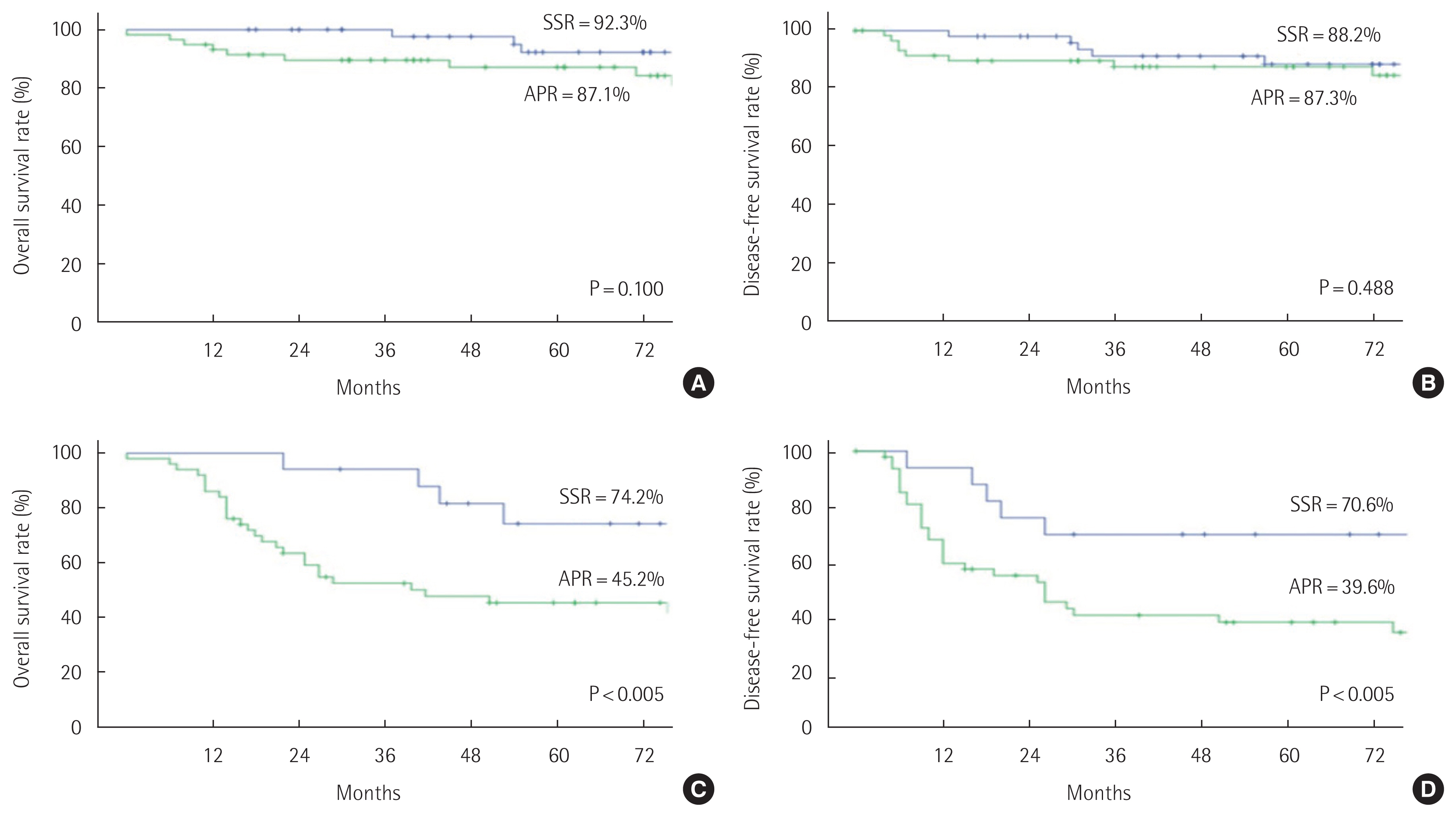
Fig. 3Oncologic outcome of ultra-low anterior resection and abdominoperineal resection groups according to chemoradiation therapy (CRTx). 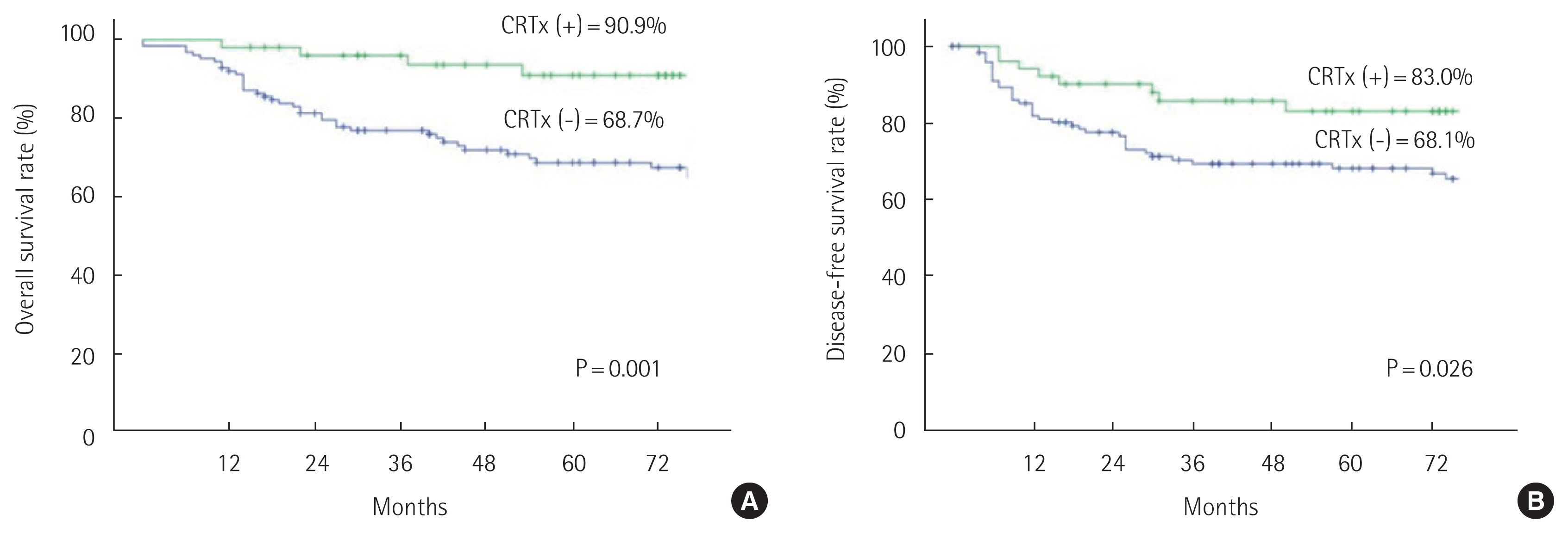
Table 1Patient characteristics (n=176) Table 2Postoperative pathologic outcomes after surgery for low rectal cancer Table 3Recurrence pattern of SSR versus APR for low rectal cancer Table 4Positive rate of pathologic lymph nodes associated with neoadjuvant CRTx Table 5Univariate and multivariate analysis for prognostic factor of disease-free survival after surgery for low lying rectal cancer |
|