INTRODUCTION
Malakoplakia is a rare chronic granulomatous disease, which was first described in 1902 by Michaelis and Gutman [1]. The pathogenesis of malakoplakia remains poorly understood, but is considered to be related to immunosuppression and/or infectious process [2]. Most reported cases of malakoplakia are associated with immunosuppressive diseases or chronic prolonged illness such as organ transplantation, tuberculosis, acquired immunodeficiency syndrome, malignancies, steroid use [3]. Originally described in the bladder, malakoplakia is most commonly found in the genitourinary tract, but can affect many systems. The gastrointestinal tract is the most common site of involvement outside of the urinary tract [3,4]. The treatment of malakoplakia varies from antibiotic treatment to surgical resection [5]. Malakoplakia shows a variable course and has a usually good prognosis, but in rare cases, can be fatal.
Herein, we present an operative case of cecal malakoplakia in a patient who has a history of operation and chemotherapy for cervical cancer. This study was approved by the Institutional Review Board of Gangnam Severance Hospital, Yonsei University, Seoul, Korea (IRB No. 3-2020-0254). Written informed consent was obtained.
CASE REPORT
A 74-year-old female patient was consulted to our department due to abdominal mass. She had a history of hypertension, diabetes mellitus and undergoing radical abdominal hysterectomy with lymph node dissection under invasive cervix cancer in a test performed for symptoms of uterine bleeding. After the operation, cervix cancer, stage IIB, was confirmed, and adjuvant concurrent chemo-radiotherapy was performed. The regimen of chemotherapy agent was used as cisplatin alone, and a total of 60.4 mg was performed 6 times based on patient body surface area (1.51 m2). Radiotherapy was also performed 28 times by 180 cGy at once (total 5,040 cGy). Cystoscopic ureteral stent indwelling was performed on left hydroureter findings for hematuria symptoms that occurred during the follow-up after adjuvant therapy. In addition, she got antibiotics therapy for cystitis and enteritis and finished concurrent chemo-radiotherapy without other complications. Afterwards, she received additional chemotherapy 4 times with paclitaxel 160 mg (110 mg/m2×1.51 m2 body surface area) and carboplatin 312 mg (4×53.03 mL/min/1.73 m2 glomerular filtration rate) at 75% dose reduction due to severe nausea symptoms. After 1 year, about 6-cm dimension infiltrative mass between the cecum base and right psoas muscle with obliteration of appendix was confirmed in computed tomography (CT) performed for follow-up (Fig. 1). We first thought of chronic inflammatory fibrotic mass related to old perforated appendix with or without appendiceal cancer with invasion to cecum base and psoas muscle. According to the patient’s past history, the possibility of metastatic cervix cancer was also considered, and the possibility of actinomycosis could not be ruled out. Magnetic resonance imaging was additionally taken to check in detail, and after confirming that there was an infiltrative lesion in the cecum base, appendiceal cancer or actinomycosis was suspected. Considering the possibility of ureter invasion before surgery, a cecectomy was planned after ureteral stent indwelling was performed on the right ureter in advance.
Severe adhesion between cecum and right psoas muscle was observed, and a hole was formed about 4 cm in the middle of the psoas muscle. The frozen biopsy of fragile adhesive tissue showed histiocytes aggregation, which was thought to be due to reactive change. Since there was no invasion with the ureter, ileocectomy was performed for palpable solid irregular mass in cecum, about 5 cm size, and the operation was ended successfully. The patient showed ileus after surgery and did not complain of any special symptoms.
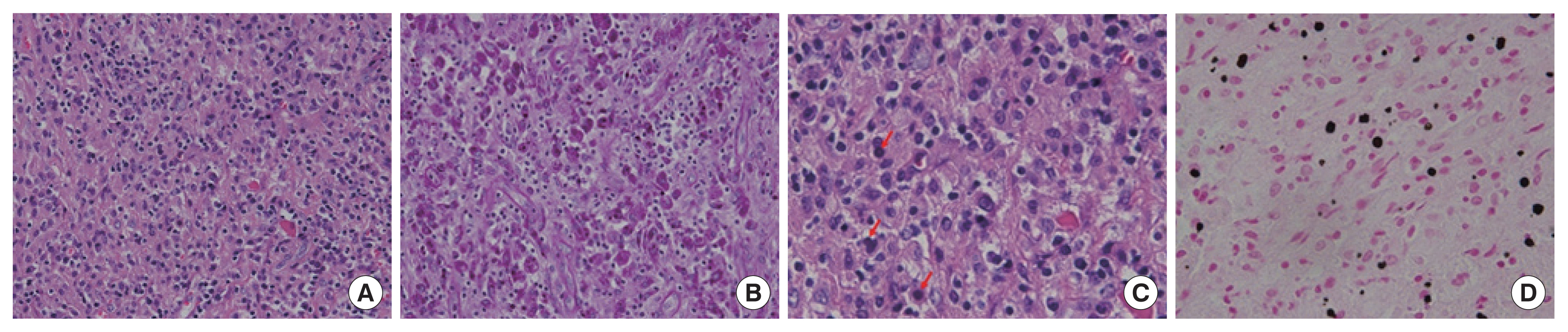
During the hospital stay, we were able to obtain a pathological examination. The gross pathologic result was appendix with periappendiceal adhesion (4.5×3.0×3.0 cm) in serosa of cecum. Dense histiocytic infiltration with Michaelis-Gutmann bodies in submucosa, proper muscle, subserosa and serosa findings was confirmed microscopically. The mass is composed of dense collection of histiocytes (Fig. 2A) showing Periodic acid–Schiff stain- positive cytoplasmic granules (Fig. 2B). Some histiocytes contain Michalis-Gutmann bodies (Fig. 2C) highlighted by von Kossa stain (Fig. 2D). This made us diagnose malakoplakia. After searching several papers, it was confirmed that antibiotics were additionally treated after malakoplakia diagnosis, and it was decided to be discharged while taking ciprofloxacin 500 mg twice in a day. Subsequently, the patient is currently being treated with outpatient clinic while maintaining the antibiotics herein.
DISCUSSION
Malakoplakia is a rare chronic granulomatous inflammatory disease described by Michaelis and Gutmann in 1902 and named by von Hansemann in 1903 [2]. The diagnosis is established histologically by the presence of large “Hansemann macrophages” containing laminated calcific spherules known as Michaelis-Gutmann inclusions [6]. The etiology and pathogenesis of malakoplakia remain unclear. But chronic bacterial infections such as Escherichia coli, Proteus mirabilis, Staphylococcus aureus, Mycobacterium tuberculosis, and Shigella boydii and impaired macrophages with an inability to completely digest and kill are related to malakoplakia pathogenesis [3]. Most reported cases of malakoplakia are associated with immunosuppressive diseases or chronic prolonged illness such as organ transplantation, tuberculosis, acquired immunodeficiency syndrome, malignancies, steroid use [3].
Treatment of malakoplakia is mostly medical treatment including antibiotics. Long-term antibiotics which work intracellularly such as quinolones, trimethoprim and rifampicin to aid the defective phagolysosomal mechanism found in malakoplakia are required [7]. And discontinuance of an immunosuppressant is needed depending on patients’ condition and morbidity. Surgical treatment indication is not defined. Surgical treatment is decided depending on the organ affected and symptoms.
Like this case, many mass-forming malakoplakia is mimic to invasive cancer, especially lesion is ulcerated or is accompanied with lymph node involvement. So, we take a review of the literature to know approach and management to mass-forming malakoplakia and found eight documented cases. Location of malakoplakia, diagnostic method and treatment were varied (Table 1). Three cases were diagnosed by fine-needle aspiration and mass was resolved after receiving medical treatment involving antibiotics and/or bethanechol without surgical treatment. Three cases took only surgical treatment and two cases were received medical treatment after en bloc resection [8–15]. In spite of small number of cases, malakoplakia was well treated by only medical treatment involving antibiotics and cholinergic drugs.
In conclusion, malakoplakia is a rare disease and has no imaging characteristic, noticing mass as malakoplakia before histological diagnosis is too hard. It is important to aware that there are lumps that resolve with medical treatment, such as malakoplakia. So if there are no restrictions, it is necessary to perform a minimal invasive biopsy such as CT-guided biopsy or fine-needle aspiration. When incidental mass was diagnosed with malakoplakia by histologically, we may try medical treatment first without surgery. At the same time, since malakoplakia is frequently accompanied by cancer, cancer evaluation work-up should be performed.