This article has been corrected. See "Erratum to: The current status of cancer survivorship care and a consideration of appropriate care model in Korea" in Volume 17 on page 57. ABSTRACTPurposeBreast cancer patients with a human epidermal growth factor receptor 2 (HER2) enriched subtype are known to have higher rates of brain metastases (BM) than other patients. This study aimed to evaluate treatment options and survival outcomes.
MethodsA total of 115 breast cancer brain metastases (BCBM) patients with nearly complete medical records were retrospectively analyzed. Additionally, 36 patients were HER2 enriched types according to histological subtypes. The BM was found by brain magnetic resonance imaging in patients who had neurologic symptoms or by regular screening. Age, breast tumor size, number of BM, histological subtypes, first treatment of breast cancer, estrogen receptor, and HER2 status, stage, local treatment of BM were analyzed. Median overall survival, 5-year survival were analyzed from the data.
ResultsThe median survival time after BM was 6 months, the mean survival time was 16.3 months, and the 5-year survival after BM was only 8.0%. Factors that significantly affect the survival of BCBM patients include histological subtype, number of BM, use of lapatinib in multivariate analysis. A total of 19 out of 36 HER2 enriched patients were treated with lapatinib or capecitabine. For the treatment of HER2 enriched patients, additional use of blood-brain barrier (BBB) crossing substances, as well as local treatment for BM, significantly improve the survival rate in the Kaplan-Meier method (P=0.001).
INTRODUCTIONBrain metastases (BM) occur in 20%–30% of patients with metastatic breast cancer and are associated with poor prognosis [1]. The American Cancer Society stated that the 5-year survival rate for patients with stage IV breast cancer is 27%. However, with the development of various treatment methods, even patients with distant metastases have significantly improved quality of life because there have been great achievements in increasing the survival rate.
Since many treatment methods for breast cancer patients with distant metastases have been developed, many studies on long-term patient survival have been conducted. However, accurate guidelines for follow-up have not been established.
Until recently, there were no Food and Drug Administration-approved treatments for BM caused by breast cancer [1], and the purpose of treatment is to alleviate symptoms or slow progression rather than attain full recovery. Current treatments for BM are surgery, whole brain radiotherapy (WBRT), stereotactic radiation surgery, chemotherapy, and targeted therapy. Recently, the incidence of BM has increased due to the increased long-term survival of patients who received more effective treatment and increased detection of brain lesions by improved imaging techniques. Previous reports confirmed that a subset of patients with triple-negative breast cancer (TNBC) or human epidermal growth factor receptor 2 (HER2) positive tumors have increased risk of developing metastatic disease in the brain [2–5]. The molecular genetic subclassification of breast cancer may be an important criterion for patient prognosis and treatment options. Oehrlich et al. [6], 2016 reported that patients with HER2 enriched cancer and TNBC are likely to have a higher risk of developing BM than estrogen receptor (ER) positive patients. BM are a major cause of morbidity in patients, leading to neurologic deficits that degrade the patient’s quality of life.
Basic systemic treatment for breast cancer and local treatment for brain lesions have been conducted; especially, the effects of trastuzumab (Herceptin) [7] and ado-trastuzumab emtansine (Kadcyla) were corroborated as compared to capecitabine-lapatinib [8,9] in HER2 enriched breast cancer patients. However, this is not yet convincing evidence in retrospective studies and more follow-up studies should be conducted.
Because drugs were developed that pass the blood-brain barrier (BBB) as a treatment for BM, several studies showed that lapatinib, a tyrosine kinase inhibitor, has an excellent effect on central nervous system (CNS) disease [10]. Therefore, in this study, we evaluated how much improvement can be achieved in the survival rate of patients with breast cancer brain metastases (BCBM) by local therapy and with combination therapy with a BBB-crossing agent for existing brain lesions.
METHODSFor human investigations, the Institutional Review Board of Chungnam National University Hospital approved our protocol in accordance with the principles outlined in the Helsinki Declaration (IRB number: CNUH 2020-04-043), and because this study was a retrospective study and did not have direct patient impact, informed written consent was not required. We conducted a retrospective study on patients with breast cancer diagnosed with BM from November 1991 to February 2015. One hundred forty-one patients diagnosed with BM were included in the study, and of that number, only those patients with brain parenchymal metastases (n=119) were selected. Four patients were lost to follow-up were excluded; therefore, there was a total of 115 patients comprising the subjects of the study. In particular, the focus was on treatment methods for HER2 enriched breast cancer patients.
BM were diagnosed by brain magnetic resonance imaging (MRI) when the patient showed neurological symptoms or symptoms were found under regular examinations. There were only three cases with histological diagnoses.
We analyzed subject age, tumor size, number of axillary lymph node metastases, initial distant metastases, stage status, tumor subtype, time interval from brain cancer to diagnosis of BM, treatment method of first systemic therapy after breast cancer diagnosis, and the number of BM.
The 5-year survival rate after BM was defined as the date from which BM were detected on the image modality (brain MRI) to 5 years of survival, and the median overall survival (OS) was defined as the date from the first diagnosis of breast cancer to the date of death.
We used the Cox regression method for univariate and multivariate survival analyses, and the Kaplan-Meier method and log-rank test to determine the difference in survival rates according to prognostic factors. When the result was less than P-value 0.05, it was considered statistically significant. The statistical analysis was conducted using SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA).
RESULTSClinicopathological characteristics of patientsThe mean OS time was 23.12 months, and the median OS time was 6 months. Clinicopathologic characteristics of a total of 115 patients with BCBM are presented in Table 1. The median age at which patients were diagnosed with breast cancer was 48.22 years (range, 27–80 years).
Of the 115 patients on study, 114 patients (99.1%) had invasive ductal carcinoma and one patient (0.9%) had an invasive micropapillary cancer. The histological subtype was hormone receptor (HR)+/HER2− (29 patients, 25.2%), HR+/HER2+ (20 patients, 17.4%), HER2 enriched (37 patients, 32.2%), and TNBC (29 patients, 25.2%). Forty-nine patients were ER positive (42.6%) and 57 patients were HER2 positive (49.6%).
The most frequently diagnosed patients with BM were at breast cancer stage II, and most of the patients underwent systemic treatment from the beginning of or after breast cancer surgery. According to the change of chemotherapy regimens, FEC (Fluorouracil, Epirubicin, Cyclophosphamide), CMF (Cyclophosphamide, Methotrexate, Fluorouracil), and AC (Adriamycin, Cyclophosphamide) were mainly used until the early 2000s, and TA (Taxane, Adriamycin), AC+T (Taxane), AC+G (Genexol) were used thereafter.
As for the first line of neo-adjuvant or adjuvant chemotherapy, 32 patients (28%) had TA, which was the most frequent, followed by AC (10.4%), AC+T (10.4%), and 10 patients with FEC (8.7%). There were many other types of chemotherapy performed, including FAC, MMM (Mitomycin, Mitoxantrone, Methotrexate), epirubicin, TAC (Taxane, Adriamycin, Cyclophosphamide), gemcitabine, etc.
The clinical characteristics and treatment of BM are summarized in Table 2. There were 26 patients with a single brain lesion and 89 patients with multiple lesions; on the other hand, extracranial metastatic sites with BM were followed by lung (n=48), bone (n=39), and liver (n=33). In addition, most patients diagnosed with BM had neurologic symptoms and symptoms such as headache, nausea, and vomiting.
As a treatment for BM, we conducted WBRT for most of the patients and followed with Gamma Knife surgery (GKS). Some patients received two or more combination treatments. At the time of diagnosis of BM, 14 patients were not treated because of a decrease of general condition, or death caused by BM and other complications. Therefore, 14 patients were not able to undergo treatment.
Survival outcomes after diagnosis of BMThe median survival after BM diagnosis was 6 months and the mean survival was 16.3 months; however, the 5-year survival rate after BM diagnosis was only 8.0%.
According to univariate analysis, factors affecting the prognosis of patients diagnosed with BM did not display significant results on initial metastases (P=0.659) or histological subtype (P=0.133). However, breast tumor size (P=0.035), operation type (P=0.023), local treatment modality for BM (P<0.001), Eastern Cooperative Oncology Group performance status (P<0.001) and number of BM (P<0.001) each were statistically significant. For multivariate analysis, treatment of BM (P=0.023) and the number of BM (P=0.002) showed significant results (Table 3).
Although no significant statistical results were observed in this study (P=0.107), as found in many previous studies, the 1-year survival rate in HR+/HER2−, HR+/HER2+, HR−/HER2+, and TNBC among subtypes was 63.2%, 35.0%, 26.8%, and 17.2%, the lowest in TNBC, respectively.
In cases of single lesion of BM, the 1-year survival rate was 66.7% and 24.5% in multiple BM, respectively. The 2-year survival rate of local treatment modality for BM was 14.8% in WBRT, 33.6% in GKS, 75% in combination therapy, 50% in surgery, and 14.3% with no treatment. As a result, the 2-year survival rate for combination therapy was the highest (P<0.001) (Fig. 1). One of four patients who received combination therapy had craniotomy treatment+ GKS and three of them received WBRT+GKS treatment.
HER2 enriched breast cancer patient groupAs mentioned earlier, the HER2 patient group, along with TNBC, is a subtype with a high risk of BM. Of the 57 patients with HER2 receptor positive (HR+/HER2+, HER2 enriched) status, 21 patients had lapatinib treatment after diagnosis of BM and showed 1-year survival rate higher than the untreated group (12-month survival rate, each 45.0% and 18.9%, respectively).
As a result of analyzing only the histological subtype HER2-enriched patients (36 of 115 patients), the characteristics of the patient group are presented in Table 4. We used the Kaplan-Meier method to compare the 2-year survival rates of lapatinib, capecitabine and combination therapy in the HER2 enriched patient group (nine patients who were treated with capecitabine and 17 untreated patients with BM that died after BM diagnosis, 25 months and 10 months, respectively).
There were no patients that were treated with lapatinib only. Capecitabine alone had a 2-year survival rate of 11.1% and 54.5% in the group treated with lapatinib+capecitabine combination therapy, respectively (P=0.001) (Fig. 2A). However, the analysis overlooked the effects of other treatments, such as other systemic chemotherapy, when one considers that extracranial metastases are already advanced in addition to BM and local therapy for BM. Therefore, it is challenging to judge the effectiveness of the BBB-crossing drug alone.
The difference in the 5-year survival rate according to the local treatment modality for BM also produced significant results (P<0.001) (Fig. 2B). The 5-year survival rate was 4.2%, 19%, 100%, 0%, and 0% according to WBRT, GKS, WBRT+GKS, surgery, and no treatment, respectively.
According to the univariate analysis of factors affecting survival rate for patients with the HER2 enriched subtype, capecitabine (hazard ratio, 0.358; 95% confidence interval [CI], 0.141–0.908; P=0.031), GKS (hazard ratio, 0.308; 95% CI, 0.115–0.828; P= 0.020), number of BM (hazard ratio, 3.777; 95% CI, 1.416–10.038; P=0.008), and operation (mastectomy; hazard ratio, 0.325; 95% CI, 0.137–0.771; P=0.011) each were found to be significant factors, and the risk of death increased by 9.839-fold when patients were not treated with local treatment for BM, and 3.777-fold with multiple BM lesions.
In multivariate analysis, among the significant factors in univariate analysis, use of BBB-crossing drugs, capecitabine (hazard ratio, 0.264; 95% CI, 0.095–0.733; P=0.011), lapatinib and capecitabine (hazard ratio, 0.120; 95% CI, 0.036–0.393; P=0.000), stage IV (hazard ratio, 6.045; 95% CI, 1.690–21.630; P=0.006) displayed significant results (Table 5). Two patients were treated with respective combination therapies among local treatments for BM, which were WBRT+GKS and craniotomy + GKS. Finally, we analyzed the last treatment as a GKS for BM because two patients survived for 5 years after treatment of BM.
Taken together, we found that a patient’s stage status (especially stage IV) and whether a BBB-crossing agent considered together is a significant combination factor that affects the patient’s clinical outcome. The number of people using BBB-crossing agents was 19 out of 36 HER2 patients: nine cases were used for system treatment by metastasis in other parts before brain metastasis, seven cases all used lapatinib+capecitabine after brain metastasis, and three cases used capecitabine only. In addition, in the HER2 patient group, patients receiving both BM local treatment (of any kind) with capecitabine treatment showed better survival rates than patients receiving BM local treatment only (P<0.001) (Fig. 2C).
DISCUSSIONBM of breast cancer often recur at the time when systemic therapy is performed for the existing breast cancer itself. In this retrospective study, only four cases of BM and breast cancer were simultaneously diagnosed at the beginning.
Because BM occurred late in the course of the disease, lack of treatment for BM was not an issue for most patients and progression of systemic disease was the main cause of death [11–13]. However, as systemic treatments have improved, control of BM is increasingly important for general disease control [14]. Local treatment is usually the first option for the treatment of BM, although the BBB may potentially undergo penetration by systemic drugs. Historically, breast cancer has been considered a relatively radiation-sensitive tumor. Therefore, WBRT is the most basic and widely practiced method for non-surgical treatment in patients with BCBM [11,12], and radiation therapy affects the survival time of patients with BM caused by breast cancer [13].
In this study, 77 of the 115 patients had WBRT and 16 patients had GKS treatment, as we compared the 5-year survival rates for local treatment modality; WBRT, GKS, combination therapy, surgery, and no treatment showed 4%, 17.9%, 37.5%, 50%, and 0% respectively (P<0.001). In the HER2 enriched patient group, the 2-year survival rates of WBRT and GKS were 8.3% and 38.1%, respectively, and one patient with WBRT+GKS survived, though one patient without treatment died at 2 years (P<0.001). In the treatment of patients with BCBM, there is a need to consider systemic treatment for the existing breast cancer in addition to local treatment for BM. Although we did not compare the group above with other HER2 enriched subtypes without BM, patients with HER2 enriched cancer or TNBC patients showed a higher risk of developing BM than the patient group of luminal subtype [15–17].
The prognosis for patients with CNS metastases is poor because the 1 year survival rate is only 20% after the first diagnosis and less than 2% after 2 years [18]. In this study, the 1-year survival rate of HER2 enriched subtype diagnosed with BM was 26.8% and the 2-year survival rate was 17%. In addition, each 1-year survival rate of HR+/HER2−, HR+/HER2+, and TNBC was 59.6%, 35%, and 17.2%, respectively, which showed a low survival rate as in other study results.
The HER2 enriched subtype has aggressive tendencies such as rapid cell proliferation, increased angiogenesis, and increased metastatic formation, though patients can survive longer as patterns of relapse and systemic disease have changed with the advent of trastuzumab. However, one-third of patients with HER2 enriched metastatic breast cancer show a tendency to experience recurrence or additional CNS disease, even though other areas of general disease are well controlled and respond well to trastuzumab treatment [19,20]. Therefore, other treatments have been developed as patients become resistant to trastuzumab.
Lapatinib is a small molecule that inhibits both EGFR (epidermal growth factor receptor) and HER2 and has high BBB penetration. Working as a single drug in the treatment of HER2 positive BM, lapatinib showed a brain response rate from 2.6% to 6% in patients with severe BM [21,22]. Compared with trastuzumab, lapatinib was limited but improved brain absorption by reaching a quarter of the brain concentration in plasma [23]; in results of a pivotal phase III trial analysis, it may reduce disease progression in the CNS [24]. Preclinical data suggest that lapatinib may act as a radiosensitizer and should possibly be administered concurrently with cranial radiation therapy [25].
Capecitabine is a cytotoxic anticancer agent which is good at BBB penetration and suitable for patients with CNS metastases, especially when tumors recur in contrast to other treatment modalities such as WBRT and intrathecal methotrexate, because it is tolerable and neurologically non-toxic. In addition, several studies report the efficacy of lapatinib+capecitabine combination therapy [22,26–28].
After trastuzumab, many treatment agents such as neratinib (Nerlynx), ado-trastuzumab emtansine (Kadcyla), and pertuzumab (Perjeta) target the HER2 receptor, and the effectiveness of the resulting survival rate has been positively demonstrated [8,9], though retrospective studies are still lacking.
Therefore, combination therapy of lapatinib and capecitabine may be considered the most appropriate agent in patients with BCBM. It showed a significantly improved survival rate in patients with lapatinib+capecitabine treatment (P=0.001).
According to an Anatolian Society of Medical Oncology (ASMO) study [29], the 1-year survival rate of WBRT and GKS in the HER2 enriched subtype group showed a higher survival rate in the patient group with BBB-crossing agents than the group patient without such drugs.
The overall prognosis after treatment of patients with BM caused by breast cancer is uncertain, thereby it has limitations such as vaguely defined selection criteria (e.g., tumor number, tumor size, lack of follow-up data, WBRT, surgery, or stereotactic radiosurgery) [30,31].
In this study, there were some limitations in how to assess the overall condition and status of a patient before and after being diagnosed with a brain metastasis. There are some cases where lapatinib or capecitabine was used as a systematic treatment before the occurrence of the brain metastasis, and there are limits that are difficult to evaluate when treating BM themselves. However, there is no question that using BBB-crossing agents with local treatment for brain metastatic lesions will improve the clinical outcomes of patients.
In the group of patients with HER2 enrichment who have a relatively high risk of BM among breast cancer patients, we determined that the use of both BBB-crossing drugs as a systemic treatment and local treatment for BM can significantly increase the survival rate of BCBM patients.
CONFLICT OF INTERESTCONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES1. Venur VA, Leone JP. Targeted therapies for brain metastases from breast cancer. Int J Mol Sci 2016;17:1543.
2. Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer 2006;107:696-704.
3. Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 2006;17:935-44.
4. Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 2008;10:R20.
5. Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 2006;24:5658-63.
6. Oehrlich NE, Spineli LM, Papendorf F, Park-Simon TW. Clinical outcome of brain metastases differs significantly among breast cancer subtypes. Oncol Lett 2017;14:194-200.
7. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92.
8. Escriva-de-Romani S, Arumi M, Bellet M, Saura C. HER2-positive breast cancer: current and new therapeutic strategies. Breast 2018;39:80-8.
9. Dieras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732-42.
10. Petrelli F, Ghidini M, Lonati V, Tomasello G, Borgonovo K, Ghilardi M, et al. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: a systematic review and pooled analysis. Eur J Cancer 2017;84:141-8.
11. Boogerd W, Vos VW, Hart AA, Baris G. Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol 1993;15:165-74.
12. Mahmoud-Ahmed AS, Suh JH, Lee SY, Crownover RL, Barnett GH. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys 2002;54:810-7.
13. Kuhnol J, Kuhnol C, Vordermark D. Radiotherapy of brain metastases from breast cancer: treatment results and prognostic factors. Oncol Lett 2016;11:3223-7.
14. Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer 2009;100:894-900.
16. Pestalozzi BC, Holmes E, de Azambuja E, Metzger-Filho O, Hogge L, Scullion M, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1–01). Lancet Oncol 2013;14:244-8.
17. Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast 2014;23:623-8.
18. Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau SW, Islam R, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 2008;19:1242-8.
19. Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003;97:2972-7.
20. Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 2004;91:639-43.
21. Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2008;26:1993-9.
22. Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 2009;15:1452-9.
23. Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsul fonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos 2008;36:695-701.
24. Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008;112:533-43.
25. Zhou H, Kim YS, Peletier A, McCall W, Earp HS, Sartor CI. Effects of the EGFR/HER2 kinase inhibitor GW572016 on EGFR- and HER2-overexpressing breast cancer cell line proliferation, radiosensitization, and resistance. Int J Radiat Oncol Biol Phys 2004;58:344-52.
26. Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013;14:64-71.
27. Sutherland S, Ashley S, Miles D, Chan S, Wardley A, Davidson N, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases: the UK experience. Br J Cancer 2010;102:995-1002.
28. Metro G, Foglietta J, Russillo M, Stocchi L, Vidiri A, Giannarelli D, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol 2011;22:625-30.
29. Cetin B, Benekli M, Oksuzoglu B, Koral L, Ulas A, Dane F, et al. Lapatinib plus capecitabine for brain metastases in patients with human epidermal growth factor receptor 2-positive advanced breast cancer: a review of the Anatolian Society of Medical Oncology (ASMO) experience. Onkologie 2012;35:740-5.
Fig. 1Five-year survival rate after brain metastases (BM) according to local treatment modality of BM. MTX, methotrexate; WBRT, whole brain radiotherapy; GKS, Gamma Knife surgery; Tx, treatment. 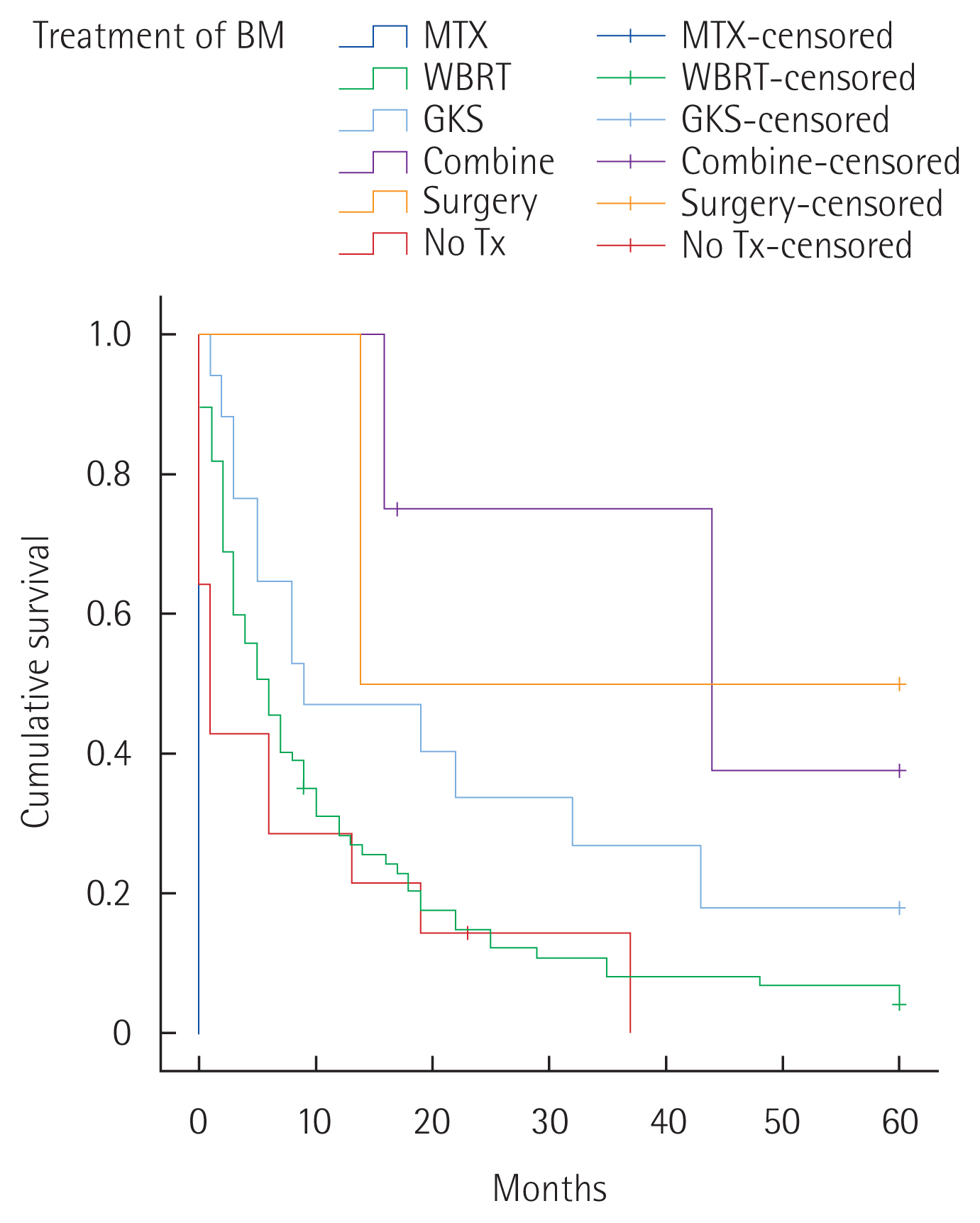
Fig. 2Five-year survival rate after brain metastases (BM) with human epidermal growth factor receptor 2 enriched breast cancer patients according to (A) use of lapatinib or capecitabine (P=0.001), (B) local treatment modality (P<0.001), (C) local treatment modality of BM and capecitabine (P<0.001). Tx, treatment; WBRT, whole brain radiotherapy; GKS, Gamma Knife surgery. 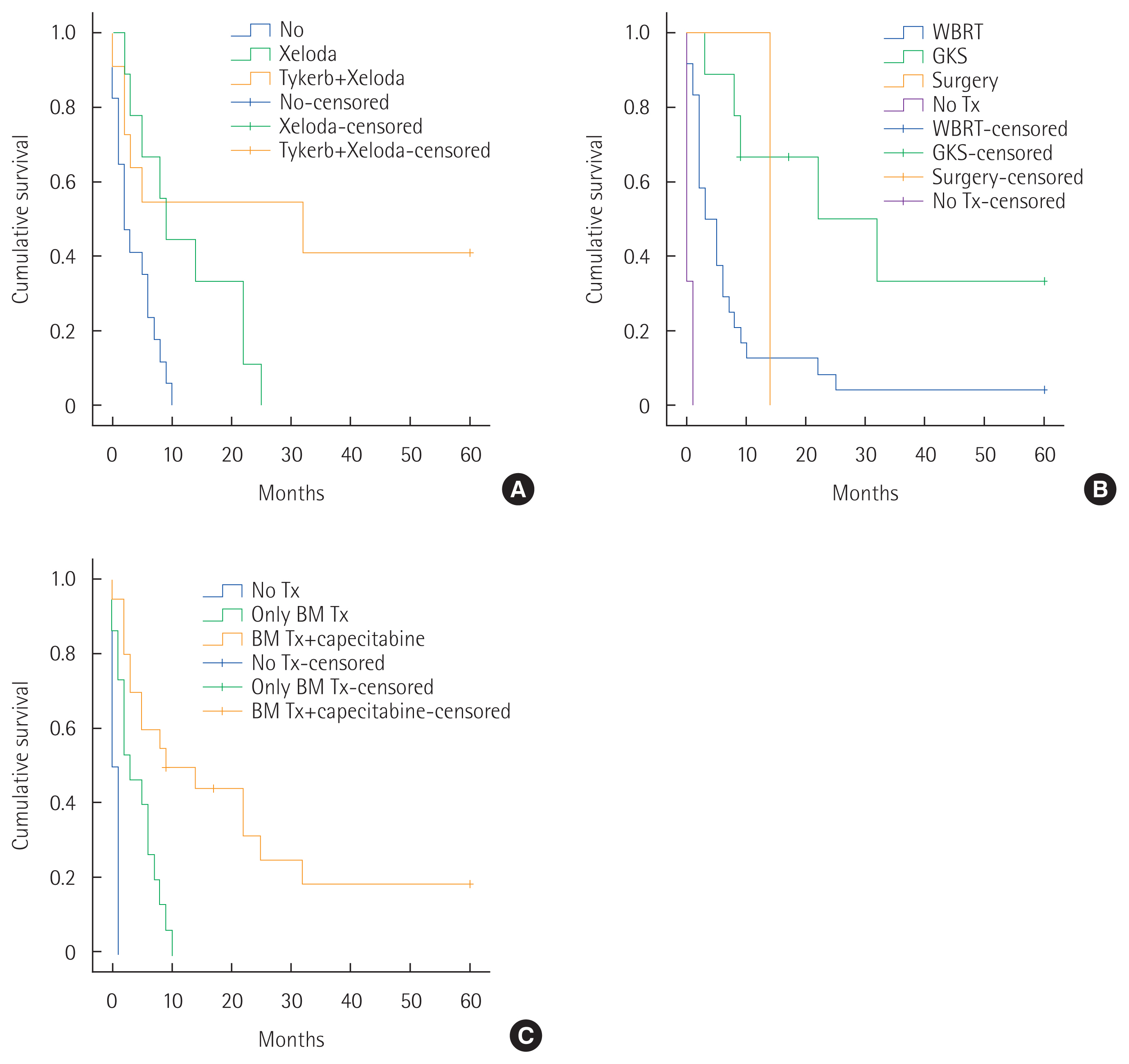
Table 1Clinicopathologic characteristics of 115 breast cancer with brain metastases Table 2Clinical characteristics and treatment of brain metastases Table 3Univariate and multivariate analysis of factors affecting 5-year survival after brain metastases
Table 4Clinicopathologic characteristics of 36 HER2 enriched breast cancer patients Table 5Univariate and multivariate analysis of factors affecting 5-year survival after BM with HER2 enriched subtype
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||