ABSTRACTPurposeThe aim of this study is to analyze the oncological outcomes of squamous cell carcinoma (SCC) of the anal canal after chemoradiation therapy (CRT) in a single institution.
MethodsFifty-one patients with anal SCC who had been treated with CRT between January 2000 and December 2010 were analyze data single center in Korea.
ResultsForty-eight patients exhibited clinical complete response. After a median follow-up of 42.1 months, 13 patients (25.5%) showed recurrence. The disease-free survival (DFS) rate was 63.4% at 5 and 10 years. The overall survival (OS) rates were 83.6% (5 years) and 75.2% (10 years). Stage I: DFS, 100%; OS, 100%; stage II: DFS, 85.7%; OS, 100%; stage IIIA: DFS, 68.6%; OS, 87.5%; stage IIIB: DFS, 34.7%; OS, 48.4%; and stage IV: DFS and OS, 0%. The local recurrence patterns were as follows: pelvic node (n=4, 7.8%), inguinal node (n=1, 2.0%), and inguinal and pelvic node (n=1, 2.0%). The systemic recurrence patterns were as follows: lung (n=2, 3.9%), para-aortic node (n=1, 2.0%), and extrapelvic site (n=2, 3.9%). N-stage represented a single independent prognostic factor for recurrence (P<0.05).
INTRODUCTIONThe incidence of anal cancer is relatively low, accounting for less than 2.5% of all gastrointestinal tract cancers [1]. Moreover, the incidence of anal cancer accounts for 0.1% of the total number of cancer patients per year in Korea [2]. The number of anal cancer patients has continually increased over the past 20 years [3-5]; risk factors reported for anal cancer are human papilloma virus (HPV), human immunodeficiency virus (HIV), men who have sex with men (MSM), smoking, chronic immunosuppression, and Crohn’s disease [6]. The median age of diagnosis for anal cancer is 60 years, and the incidence in females is slightly higher than that in males [7]. The most common histological type of anal cancer is squamous cell carcinoma (SCC); other rare histological types are adenocarcinoma, melanoma, sarcoma, and neuroendocrine tumor.
The standard treatment for anal cancer was abdominoperineal resection (APR) before the introduction of chemoradiation therapy (CRT) [8]. However, since concurrent CRT for anal cancer was introduced by Nigro et al. [9] in 1974, CRT has become the standard therapy for SCC in anal cancer. CRT for anal cancer has statistically significantly improved the overall survival (OS) rate, colostomy-free survival rate, and reduced the nodal recurrence rate [10]. The aim of this study is to present experiences with CRT for SCC in anal cancer at a single center in Korea.
METHODSFifty-one patients diagnosed with SCC in anal cancer and treated with CRT at a single center in Korea between January 2000 and December 2010 were prospectively recorded in a database. This included information on demographics, staging, response to treatment, and recurrence (local/distant). Routine HIV testing or HPV typing were not carried out for any of our patients. All patients had their diagnosis confirmed with biopsy and underwent staging with chest, abdomen, and pelvis computed tomography (CT), and pelvis magnetic resonance imaging (MRI). Inguinal node involvement was based on clinical or radiological results up until 3 years ago when the policy to perform fine needle aspiration or cut biopsy of palpable lymph nodes was introduced. All patients were discussed at multidisciplinary meetings.
Patients were assessed at 6 weeks to 3 months for response and significant treatment-related complications were recorded. Response to therapy was classifiedas complete response (CR), partial response (PR), static/stable disease (SD), or progressive disease (PD). Patients were closely followed up every 3 months for 2 years and 6 months for another 3 years. CT scans were performed annually or as and when required. Patients presenting with locoregional recurrence underwent salvage therapy if they had resectable status. The median total radiation dose was 5,300 cGy (range, 4,320–6,300 cGy). The concurrent chemotherapy regimens were fluorouracil (5-FU)/mitomycin (MMC) or 5-FU/cisplatin (DDP). Patients received two 4-day cycles of continuous intravenous infusion of 5-FU (1,000 mg/m2/day) combined with bolus intravenous MMC (10 mg/m2) or DDP (100 mg/m2) on the first day of each cycle.
Statistical analysisData were analyzed using the SPSS statistical program (PASW ver. 18 for Windows, SPSS Inc., Chicago, IL, USA). Cumulative-incidence methods were used to estimate the rate of cancer recurrence. OS and disease-free survival (DFS) were analyzed using the Kaplan-Meier method. Prognostic factors for recurrence were assessed for statistical significance using the log rank test and Cox analysis method. P-values of less than 0.05 were considered statistically significant.
RESULTSPatient characteristicsThe median age was 61.8 years (range, 38–83 years). The sex distribution was 20 male (39.2%) and 31 female (60.8%). The median weight was 59.3 kg (range, 40–82 kg). The median body mass index (BMI) was 23.8 kg/m2 (range, 17.3–28.5 kg/m2). The American Joint Committee on Cancer (AJCC) T-stage distribution was as follows: T1 (≤2 cm), 14 patients (27.5%); T2 (2–5 cm), 19 patients (37.3%); T3 (≥5 cm), 6 patients (11.8%); and T4 (invades adjacent organs), 12 patients (23.5%). The N-stage distribution was as follows: N0, 25 patients (49.0%); N1 (metastasis in perirectal lymph node), 15 patients (29.4%); N2 (metastasis in unilateral internal iliac and/or inguinal node), 5 patients (9.8%), and N3 (metastasis in perirectal and inguinal lymph node and/or bilateral internal iliac and/or inguinal lymph node), 6 patients (11.8%). In terms of the clinical stage of all patients, there were 9 patients (17.6%) with stage I cancer; 14 patients (27.5%) with stage II; 10 patients (19.6%) with stage IIIA; 16patients (31.4%) with stage IIIB; and 2 patients (3.9%) with stage IV. In terms of response after CRT, it was recorded that 48 patients (94.1%) exhibited clinical complete response and 3 (5.9%) showed partial response (Table 1).
Complications of CRT for anal cancer patientsAfter CRT, three patients (5.9%) complained of anal pain, and 1 patient (2.0%) developed a rectovaginal fistula. Meanwhile, 2 patients (3.9%) had radiation proctocolitis, and 1 (2.0%) was treated for neutropenic sepsis (Table 2).
Oncological outcomesThe DFS rate was 63.4% after both 5 and 10 years (Fig. 1). The 5- and 10-year OS rates were 83.6% and 75.2%, respectively (Fig. 2). DFS and OS were both 100% for stage I, 85.7% and 100% for stage II, 68.6% and 87.5% for stage IIIA, 34.7% and 48.4% for stage IIIB, and both 0% at stage IV (2 patients) (Figs. 3, 4). Univariate analysis for the prognostic factor of the recurrence of anal cancer after CRT showed that tumor size (≥5 cm; P<0.006), and node metastasis (P<0.014), and age (≥55 years; P<0.031) significantly influenced oncological outcomes (Table 3). On multivariate analysis, the N-stage was the single independent prognostic factor for recurrence (P<0.05).
Patterns of recurrenceThe total number of patients with recurrence was 13 (25.5%). Local recurrence was found in 6 patients (11.8%) and systemic recurrence in 7 patients (13.7%). The local recurrence patterns were as follows: pelvic node (n=4, 7.8%), inguinal node (n=1, 2.0%), and inguinal and pelvic node (n=1, 2.0%). The systemic recurrence patterns were as follows: lung (n=3, 5.9%); para-aortic node (n=1, 2.0%), and extrapelvic site (prostate, vulva, perineum: n=3, 5.9%) (Table 4). Treatments for patients with recurrence involved three salvage operations, five cases of systemic chemotherapy, and three cases of CRT. Two patients refused additional treatment.
DISCUSSIONThe most common presenting symptoms of anal cancer are bleeding and anal pain. Other symptoms are palpable lumps, pruritus, discharge, tenesmus, changes in bowel habits, fecal incontinence, and rarely, inguinal lymphadenopathy. The etiological risk factors of anal cancer are smoking [11], HPV infection [12], sexual practices (anal intercourse, multiple sex partners, men who have sex with men [13], HIV-positive status [14], receipt of organ transplants [15], and a history of cervical cancer in women [16]. The most important etiological factor for anal cancer is infection with HPV (HPV-16 and HPV-18) [12]. Patients with HIV are less likely to achieve a complete clinical response to treatment, are more likely to die of their cancer, and have a significantly shorter median time to cancer death [17]. We did not perform HIV assessment or HPV typing in patients with anal cancer on a routine basis, although these measures have well-documented effects on therapy and prognosis. This is because the incidence of HIV infection is low in Korea (0.003%–0.01%) [18].
Squamous carcinomas are radiosensitive, and anal cancer radiotherapy with radiosensitizers was first attempted by Nigro et al. [9] in 1974. Since the introduction of Nigro’s regimen, chemoradiation (5-FU/MMC) has predominantly been used for the treatment of anal cancer. A United Kingdom Coordinating Committee on Cancer Research (UKCCR) trial [19] reported that the 3-year locoregional failure was 39% in anal cancer patients after CRT. The European Organization for Research and Treatment of Cancer (EORTC) trial [20] reported a 5-year survival rate of 56% and locoregional failure of 32% in anal cancer patients after CRT. The RTOG 87–04 trial [21] reported locoregional failure of 16% and a 4-year OS rate of 76% in 146 anal cancer patients. The RTOG 98–11 trial [22] reported locoregional failure of 25% and a 5-year survival rate of 84% in 322 anal cancer patients. The Anal Cancer Trial II (ACT II) trial [23] reported a CR rate of 90.5% and locoregional failure of 26% in 3 years (Table 5). Moreover, the Korea Central Cancer Registry (KCCR) reported a 5-year OS rate of 74.9% in 2006–2010 [24]. Trials like the UKCCR [19] and EORTC [20] trials also confirmed superiority of chemoradiation in combination over radiation alone. Furthermore, according to the recent ACT II trial [23], there was no difference in response or survival rates between mitomycin C versus cisplatin. This was based on increased CR to chemoradiation, decrease stoma rates, and lowered recurrence rates. The current NCCN guidelines recommend 5-FU with mitomycin C with concurrent radiotherapy for all localized anal canal cancer and 5-FU with cisplatin with concurrent radiotherapy is recommended for widely metastatic disease [25].
In the present study, the CR rate was 94.1%, and the 5-year OS rate was 83.6%. The oncological outcomes of the present study were comparable to those in the previous literature (Table 5). In particular, for the oncological outcomes related to the specific stages, we found that the outcomes of stage III showed sharp decreases (68.6% and 87.5% for stage IIIA, 34.7% and 48.4% for stage IIIB) compared to stage I/II. Stage III anal cancer involves node metastasis and enlarged tumor size. In the present study, the three significant factors that were found to influence oncologic outcomes via univariate analysis were tumor size (≥5 cm; P< 0.006), and node metastasis (P<0.014), and age (≥55 years; P<0.031). On multivariate analysis however, the N-stage was the single independent prognostic variable for recurrence (P<0.05). N-stage and nodal involvement are important for the oncologic outcomes of anal cancer.
The UKCCR trial [19] included anal margin cancers (25%) other than those with local excision, while the EORTC trial [20] excluded T1/T2 cancers that were node negative. The present study included only SCC in anal cancer and excluded anal margin cancers. Thus, we hope that the present study would provide outcomes for subtypes of anal cancer despite it being a small-sized retrospective study.
The treatment of patients with PR after CRT was different from that reported in previous trials. In the UKCCR trial, patients who had>50% response after CRT were given boost radiotherapy, while patients with <50% response underwent salvage operation and colostomy. However, in the EORTC trial, patients with SD or PD underwent salvage operation, while patients with PR and CR were given boost radiotherapy. Moreover, in RTOG 87–04, all patients with residual disease were given boost radiotherapy along with chemotherapy (cisplatin) and underwent salvage operation if there was still no response after another 6 weeks. However, we did not have a proper protocol treatment for anal cancer patients according to the response rate after CRT, so this limited the present study. More studies are required, along with a plan to establish a proper protocol for treatment according to the response rate after CRT.
Treatment for recurrent anal cancer mainly involves surgical therapy. The recurrence of anal cancer requiring salvage APR is evident in approximately 30% of cases [26]. The outcome of salvage APR was reported to be 30%–77% in a 5-year local regional control study [27,28]. In the present research, salvage APR was performed in three patients among six with local recurrence. They all survived throughout the follow-up period.
The present study showed that combination CRT for SCC in the anal canal is effective for improved oncological outcomes. In particular, it was extremely effective in the early stage of anal canal cancer. However, the advanced stage of anal canal cancer with nodal metastasis showed poor oncological outcomes. Initial nodal status may influence oncological outcomes. A multimodal approach may be more appropriate for advance stages of anal canal cancer.
REFERENCES2. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 2013;45:1-14.
3. Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer 2004;101:281-8.
4. Brewster DH, Bhatti LA. Increasing incidence of squamous cell carcinoma of the anus in Scotland, 1975-2002. Br J Cancer 2006;95:87-90.
5. Nielsen A, Munk C, Kjaer SK. Trends in incidence of anal cancer and high-grade anal intraepithelial neoplasia in Denmark, 1978-2008. Int J Cancer 2012;130:1168-73.
7. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2008. Bethesda (MD): National Cancer Institute;2011 [cited 2016 May 26]. Available from: http://seer.cancer.gov/archive/csr/1975_2008/.
8. Lopez MJ, Bliss DP Jr, Kraybill WG, Soybel DI. Carcinoma of the anal region. Curr Probl Surg 1989;26:525-600.
9. Nigro ND, Vaitkevicius VK, Considine B Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 1974;17:354-6.
10. De Bari B, Buglione M, Maddalo M, Lestrade L, Spiazzi L, Vitali P, et al. External beam radiotherapy +/- chemotherapy in the treatment of anal canal cancer: a single-institute long-term experience on 100 patients. Cancer Invest 2014;32:248-55.
11. Daling JR, Sherman KJ, Hislop TG, Maden C, Mandelson MT, Beckmann AM, et al. Cigarette smoking and the risk of anogenital cancer. Am J Epidemiol 1992;135:180-9.
12. Chin-Hong PV, Palefsky JM. Natural history and clinical management of anal human papillomavirus disease in men and women infected with human immunodeficiency virus. Clin Infect Dis 2002;35:1127-34.
13. Daling JR, Weiss NS, Hislop TG, Maden C, Coates RJ, Sherman KJ, et al. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med 1987;317:973-7.
14. Bower M, Powles T, Newsom-Davis T, Thirlwell C, Stebbing J, Mandalia S, et al. HIV-associated anal cancer: has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr 2004;37:1563-5.
15. Hemke AC, Dekker FW, Bos WJ, Krediet RT, Heemskerk MB, Hoitsma AJ. Causes of decreased use of peritoneal dialysis as a kidney replacement therapy in the Netherlands. Ned Tijdschr Geneeskd 2012;156:A3871.
16. Edgren G, Sparen P. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: a prospective population-based study. Lancet Oncol 2007;8:311-6.
17. Kim JH, Sarani B, Orkin BA, Young HA, White J, Tannebaum I, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum 2001;44:1496-502.
18. Choe KW. Epidemiology of HIV/AIDS: current status, trend and prospect. J Korean Med Assoc 2007;50:296-302.
19. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996;348:1049-54.
20. Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9.
21. Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 1996;14:2527-39.
22. Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB 3rd, Thomas CR Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914-21.
23. James RD, Glynne-Jones R, Meadows HM, Cunningham D, Myint AS, Saunders MP, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2×2 factorial trial. Lancet Oncol 2013;14:516-24.
24. Park HC, Jung KW, Kim BW, Shin A, Won YJ, Oh JH, et al. Characteristics and survival of Korean anal cancer from the Korea central cancer registry data. Ann Coloproctol 2013;29:182-5.
25. NCCN Clinical practice guidelines in oncology (NCCN Guidelines) Anal Carcinoma Version 2 [Internet]. Washington, PA: National Comprehensive Cancer Network;2014 [cited 2014 Fed 26]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/anal.pdf.
26. Goto H, Ikenaga M, Yasui M, Miyazaki M, Mishima H, Tsujie M, et al. A case of salvage treatment for local recurrence of squamous cell anal carcinoma after chemoradiation. Gan To Kagaku Ryoho 2010;37:2659-61.
Fig. 1.Disease free survival rate foranal cancer with chemoradiation therapy. DFS, disease-free survival. 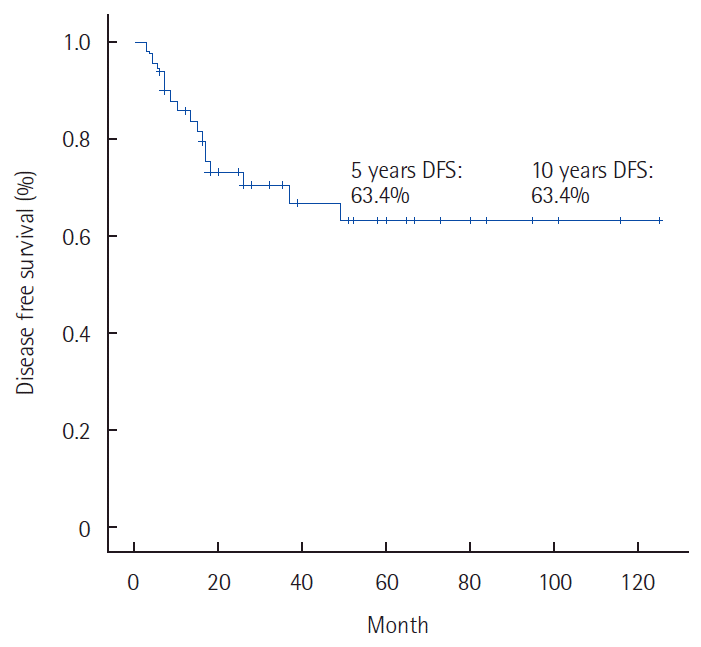
Table 1.Characteristics of patients Table 2.Complications of chemoradiation treatment for anal canal cancer
Table 3.Univariate analysis to prognostic factor for recurrence of anal cancer after chmoradiationtherapy Table 4.Patterns of recurrence of anal canal cancer Table 5.Comparing oncological outcomes with literature
UKCCR, United Kingdom Coordinating Committee on Cancer Research; EORTC, European Organization for Research and Treatment of Cancer; RTOG, Radiation Therapy Oncology Group; ACT II, Anal Cancer Trial II; CTRT, chemo therapy and radiation therapy; 5-FU/MMC, fluorouracil/mitomycin; CR, complete response. |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||