INTRODUCTION
Choriocarcinoma is a germ cell tumor belonging to the spectrum of gestational trophoblastic diseases [1,2]. It is characterized by wide and rapid hematogenous metastases, usually to the lungs and vagina [3]. It mostly occurs in the gonads and less frequently in extragonadal locations, including the mediastinum, retroperitoneum, stomach, or liver as a primary tumor. Primary choriocarcinoma of the mediastinum or stomach is more frequently reported than primary hepatic choriocarcinoma. To our knowledge, nine case reports of primary hepatic choriocarcinoma have been published in international journals [4-8]. In this case report, we describe a patient with primary liver choriocarcinoma and lung metastases.
CASE REPORT
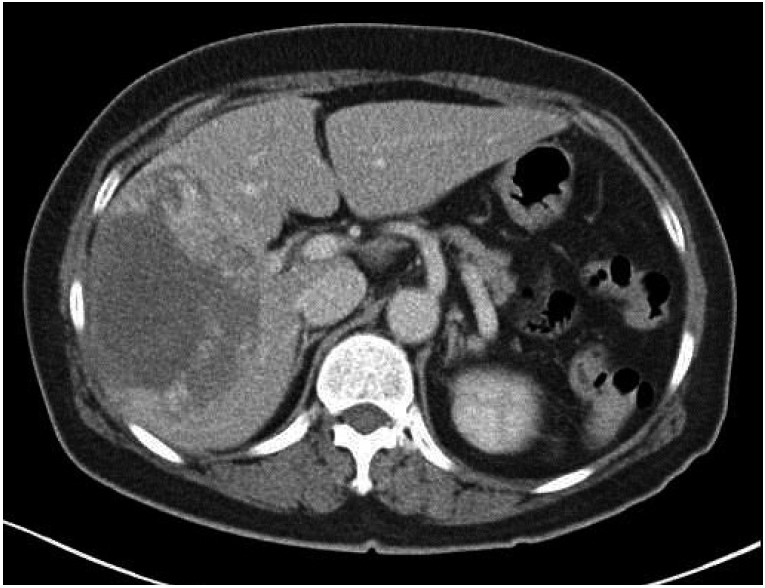
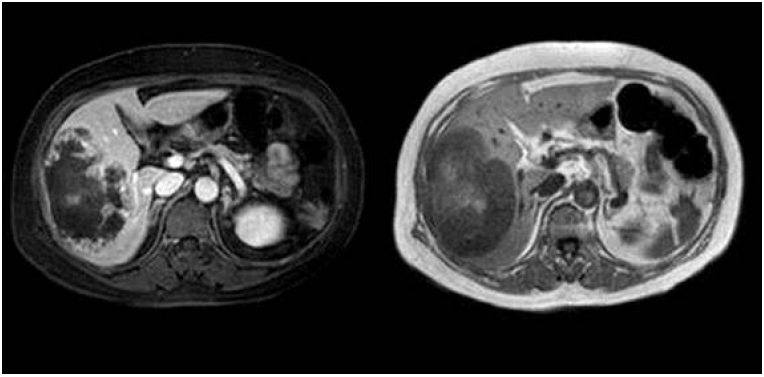
A 59-year-old woman was referred to our clinic with a history of upper right quadrant pain lasting 1 month. The patient’s medical history was unremarkable. Computed tomography (CT) revealed a large hypervascular mass in the right liver (Fig. 1). Magnetic resonance imaging revealed large masses with tiny daughter nodules in the right lobe of the liver, characteristic of angiosarcoma (Fig. 2). Cervicovaginal smear was negative for intraepithelial lesions and malignancy. Blood tests on admission revealed a low hemoglobin level (6.5 g/dL).
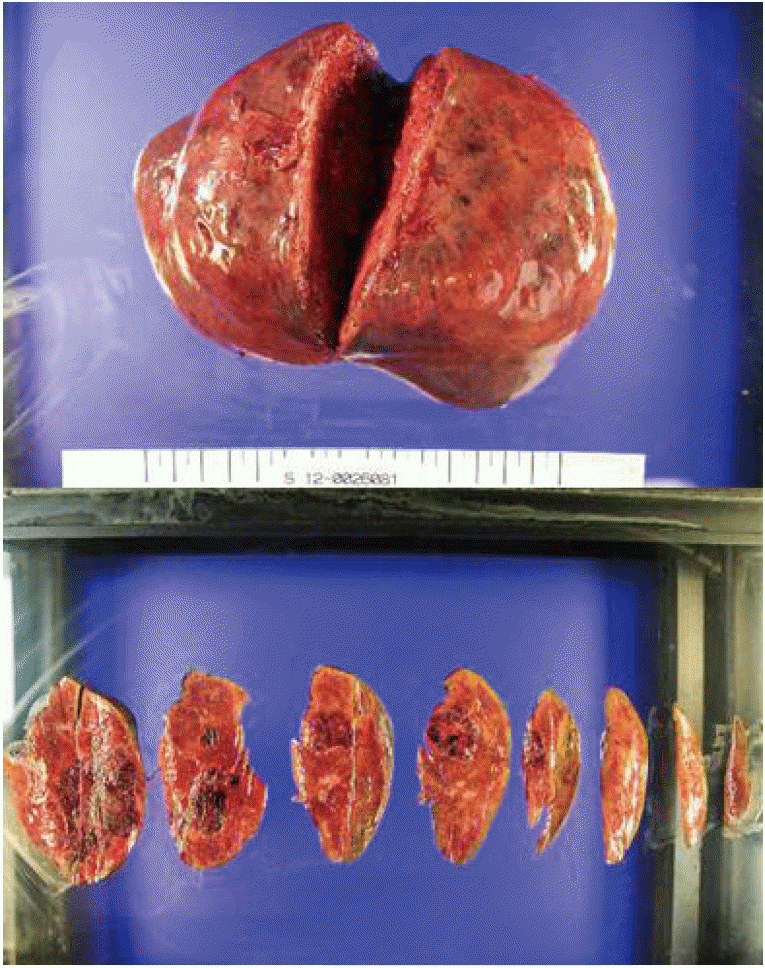
The patient underwent right hepatectomy (Fig. 3). At surgery, the tumor was attached to the peritoneum but was easily dissected without leaving residual tumor tissue. The patient recovered without immediate postoperative complications. However, on postoperative day 6, the patient developed vaginal spotting. Pancreaticobiliary CT revealed no significant abnormality, but several pulmonary nodules were found, probably representing pulmonary metastasis. Chest CT revealed multiple round nodules in both lungs, most likely metastases. The nodules were well defined, of variable size, and were randomly distributed. On postoperative day 12, positron-emission tomography confirmed the presence of multiple metastatic nodules in both lungs.
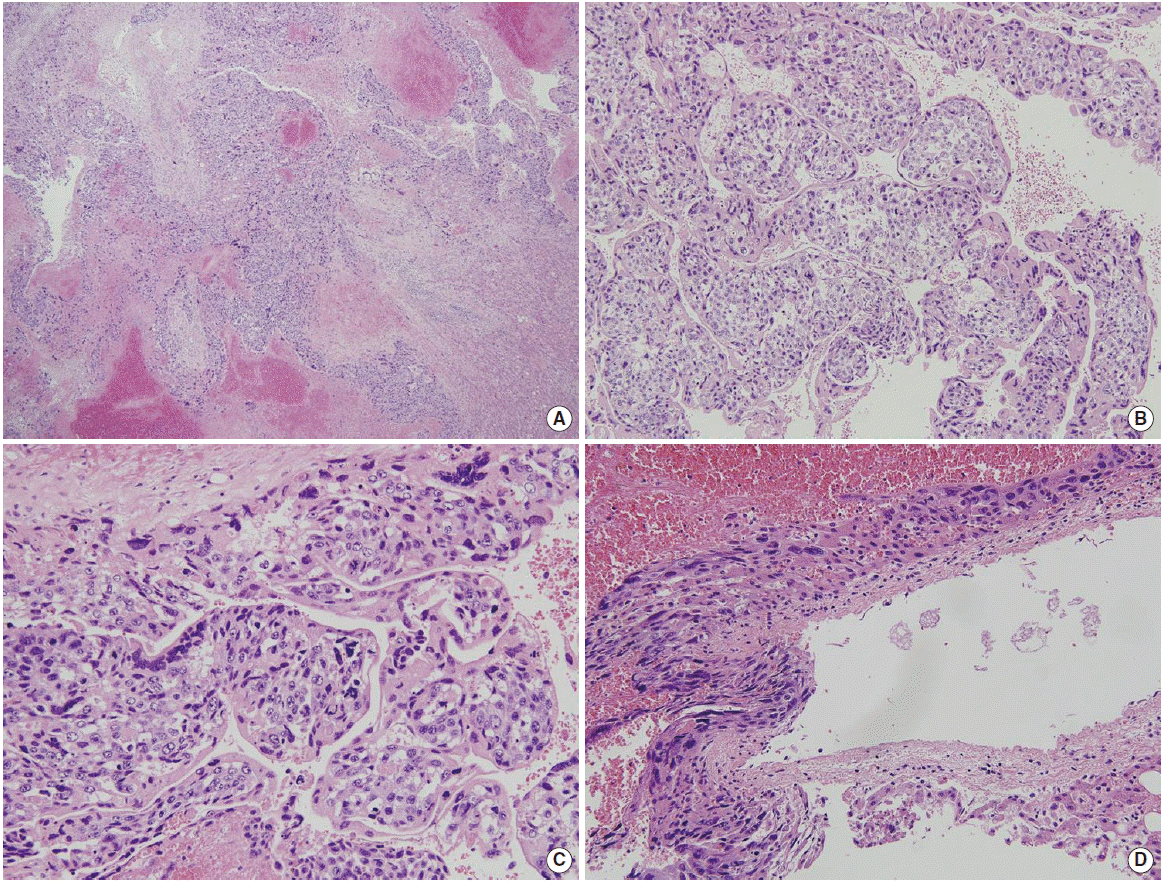
Histopathology revealed the tumor as a choriocarcinoma containing numerous syncytiotrophoblasts (Fig. 4). Immunohistochemistry was positive for β-human chorionic gonadotropin (hCG; Fig. 5) and CK-19, with a Ki-67 labeling index of 90%. The tumor was also focally positive for CD31 and human placental lactogen. The patient started chemotherapy (vincristine and cyclophosphamide) on postoperative day 16. Serum and urine hCG levels (checked after we received the pathologic report) were very high (1,637 mIU/mL and 3,070 mIU/mL, respectively). In contrast, α-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19-9 levels were within normal ranges. The patient’s clinical condition improved, and the patient was discharged on postoperative day 17. On postoperative day 29, her serum hCG level had fallen to 210 mIU/mL, but was still relatively high. The patient is currently being followed up at our outpatient department.
DISCUSSION
Primary hepatic choriocarcinoma is a rare malignant tumor with propensity for rapid metastasis. Most choriocarcinomas located in the liver are of an infantile type, indicative of metastasis from occult placental choriocarcinoma [9,10]. All of the patients in previous reports were males [4,6,7]. Case reports of primary hepatic choriocarcinoma in females are difficult to find.
A review of the literature and our experience with the present case confirmed the difficulty of diagnosing hepatic choriocarcinoma. Percutaneous needle biopsy is technically challenging and is not recommended because of the rarity of the tumor and the risk of post-biopsy bleeding [7]. Detection of overproduction of the tumor marker hCG can help with diagnosis, but hCG levels are not routinely measured in the diagnosis of liver tumors. In our patient, hCG levels were only measured postoperatively after histopathology had confirmed the presence of choriocarcinoma. For these reasons, the diagnosis of primary hepatic choriocarcinoma can only be confirmed after histopathologic testing of the resected tumor or during autopsy.
Another major issue is the differential diagnosis of primary hepatic choriocarcinoma from metastasis from other sites, especially from the testis or ovary. This is confounded by the fact that primary gonadal tumors can spontaneously regress, such that only distant metastasis can be detected in the patient [11-13]. In all of the previously reported cases, the diagnosis of primary hepatic choriocarcinoma was confirmed after examination of serial pathologic sections of the gonads during autopsy to exclude the possibility of a tumor or a tumor scar in these tissues. This method is considered a specific criterion for the diagnosis of extragonadal choriocarcinoma [14]. Another potential diagnostic method is celiac angiography, which can detect hypervascular hepatic masses with aneurismal dilatation of the peripheral hepatic arteries in the arterial phase and persistent vascular lakes in the venous phase [15].
The treatment of choice for patients without metastasis is surgical resection followed by chemotherapy. In an earlier study, the response to chemotherapy was poor, and the median survival time was only 2–8 months [7]. Our patient was discharged on postoperative day 17. She is currently undergoing chemotherapy in our outpatient department. The patient is still alive and asymptomatic 7 months after surgery.
In conclusion, primary hepatic choriocarcinoma is an aggressive tumor in adults that is extremely rare, especially in females. Further studies are needed to improve the diagnosis and treatment of this tumor.