INTRODUCTION
Gastrointestinal stromal tumor (GIST) is one of the most commonly found mesenchymal tumors in the gastrointestinal (GI) tract. It expresses the KIT protein. The term GIST was first used by Mazur and Clark [1] in 1983 and now it’s defined as cellular spindle cell and epithelioid, or pleomorphic mesenchymal tumors of the GI tract [2,3].
Gastrointestinal autonomic nerve tumor (GANT) is a subset of GIST and a very rare mesenchymal tumor with neuronal differentiation [2]. GANT was first reported in 1984 by Herrera et al. [4] as plexosarcomas. Walker and Dvorak identified ultrastructural evidence for newly recognized entity of GANTs [5]. GANT in the rectum was documented fully with clinical information for the first time by Lev et al. [6]. We report here our experience in treating a 34-year-old female patient with GANT in the lower rectum.
CASE REPORT
A 34-year-old female was referred to our hospital because of several episodes of painless rectal bleeding. She; however, did not complain of abdominal pain, constipation or any other significant symptoms. She had no past medical history of neufibromatosis or endocrine abnormality and had undergone a cesarean section 3 years prior to this visit.
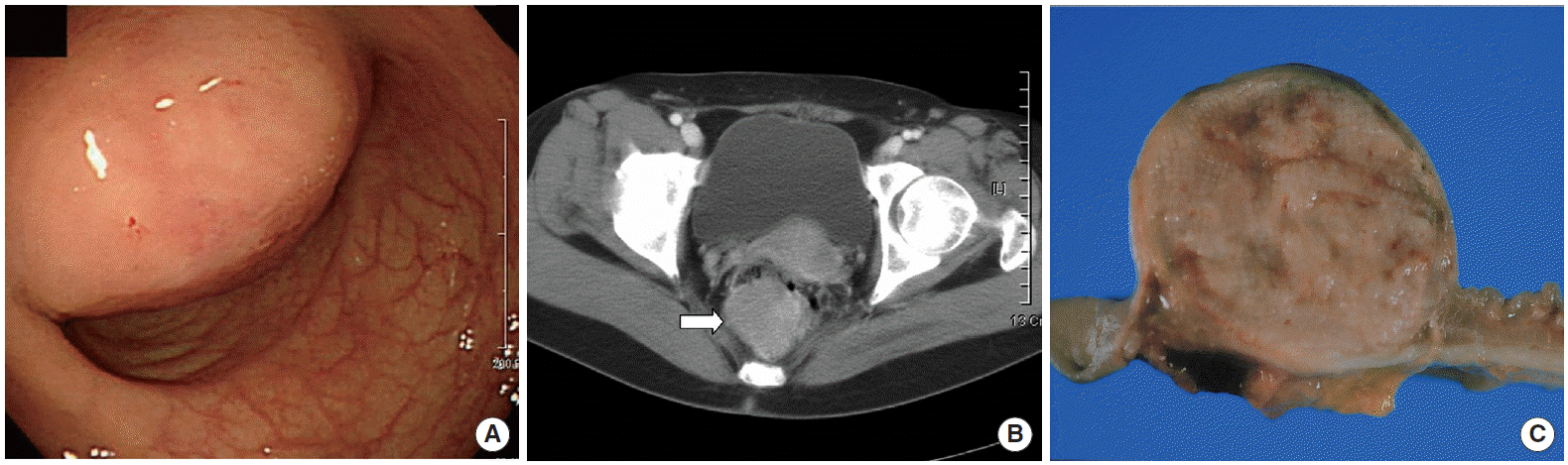
On digital rectal examination, a round and smooth submucosal tumor was palpated at posterior wall of the rectum, approximately 5 cm above the anal verge. The mass was not fixed and its diameter was approximately 5 cm. Laboratory data revealed a hemoglobin of 9.6 g/dL and normal serum levels of carcinoembryonic antigen, white blood cell and platelet count. In colonoscopy, protruded mass was covered with focally erosive epithelium in the lower rectum (Fig. 1A). Abdomino-pelvic computed tomography (CT) scan showed a 5 cm enhancing rectal mass without enlarged perirectal lymph nodes (Fig. 1B). Core needle biopsy was performed. Given the impression of GIST, it was assumed that the patient underwent low anterior resection with double stapling technique. A well-demarcated submucosal tumor was soft and located in the lower rectum.
Macroscopically, the tumor was 5×4×3.5 cm in size and its mucosa had focal ulceration. The cut surface was homogenous and yellow-grayish colored. Hemorrhagic necrosis was not found (Fig. 1C). Microscopically, the tumor was composed of polygonal cells and spindle cells. The tumor cells contained plump eosinophilic cytoplasm and eccentrically located large round vesicular nuclei with prominent nucleoli (Fig. 2A). There were no mitotic figures per 50 high power fields (HPF) and no lymph node metastasis. Surgical resection margins were free of tumor. Immunohistochemistry results are shown in Fig. 2B, C, and Table 1. Myoglobin staining was unrewarded and Ki-67 index was less than 1%. Electron microscopy (EM) revealed skeinoid fibers (Fig. 2D). Diagnosis of GANT was based on histological, immunohistochemical staining, and EM. The patient did not receive any adjuvant therapy and was discharged on the tenth postoperative day without complications.
DISCUSSION
GIST is a rare form of tumor in the GI tract, even in the omentum and mesentery [2,7]. GIST is mostly found in the stomach and small intestine. Only approximately 5% of all GISTs occur in (or “develop from”) the rectum [8]. As immunohistochemical and ultrastructural studies have developed, GISTs have been divided into several phenotypic groups.
GANT, a subset of GIST, demonstrates ultrastructural features of the autonomic nerve phenotype [3,9,10]. To the best of our knowledge, there are only a few reported cases of GANTs in the rectum. Predominant sites of the tumor are small intestine and stomach. Occasionally GANTs are found in the esophagus, colon and rectum, retroperitoneum, omentum, mesentery, and peritoneum [9,11-13]. Due to the rarity of this neoplasm, clinical features and biological behaviors of GANTs have not been fully understood.
Most patients who develop GANT are between the ages of 10 and 87, with slight male predominance. It usually accompanies abdominal pain, swelling, GI bleeding, and vomiting [5,6,9-13]. Our GANT patient had rectal bleeding with minor ulceration on the mucosa of the tumor. Endoscopic examination of the tumor may show smooth protrusion of the bowel wall having bleeding or ulceration mucosa [10]. CT scans can reveal location, size, primary tumor extension, and the presence of metastatic lesion.
Immunohistochemistry and EM can provide further supportive evidence of GANTs [6,9-11,13]. The tumors are stained consistently positive for vimentin and neuron-specific enolase. Some may also be stained positive for synaptophysin, S-100, neurofilaments, vasoactive intestinal peptides, and chromogranin. It’s stained negative for cytokeratin, muscle specific actin, CD34, desmin, serotonin, and glial fibrillary acid protein. The tumor we report was composed of plump eosinophilic cytoplasm and was stained positive for vimentin, c-kit, neuron specific enolase, S-100, neurofilaments, synaptophysin, and smooth muscle actin. Diagnostic ultrastructural features include neuro-scretory granules, neurotubles, skeinoid fibers and the interdigitating complex cellular processes joined by rudimentary cell junctions [10-13]. Initially, GANT was regarded as malignant with poor prognosis [4]. This view was later challenged by Lee et al. [12], who suggested GANT was not always malignant. Histology won’t give easy prediction of prognosis, though certain features can be predictive of malignant behavior: tumor size of >5 cm, infiltration of adjacent structures, presence of necrosis, mitotic rate >1–10 HPF, high nuclear cytoplasmic ratios, and infiltration of the mucosa overlying the lesion [6]. In 2002, risk categories based on the size of GIST and mitotic counts were introduced [3]. Tumor size of >5 cm and mitotic rate of >5/50 HPF indicate high potential for malignancy. The tumor we report was less than 5 cm in size and had no mitotic figures on 50 HPF. Generally, metastatic patterns of GANTs are still unclear.
A major treatment modality is radical surgical resection with free margins [6,10-12], though it’s hard to establish a definitive treatment plan due to the low incidence of GANT. Most patients initially go through surgery, which is potentially curative excision or palliative debulking procedure when the former is not feasible [11]. There is no clear evidence about the role of adjuvant and palliative modalities [11,13]. GANT, a subset of GIST, is a very rare tumor in the GI tract, especially in the rectum. Its clinical features and biological behaviors still remain unclear. Further research is needed to establish proper treatment.