ABSTRACTPurpose:This study aimed to compare expression of human epidermal growth factor receptor (HER) family and insulin-like growth factor-1 receptor (IGF-1R) before and after targeted therapy in HER-2 positive breast cancers.
Methods:Epidermal growth factor receptor (EGFR), HER-3, HER-4, and IGF-1R were immunohistochemically determined using pairwise archives tumor blocks of 28 patients received chemotherapy and HER-2 directed therapy between January 2007 and December 2011. Of them, 5 good responders achieved a pathologic complete response after neoadjuvant therapy and 23 poor responders experienced disease progression or metastasis even HER-2 targeted therapy. Expression of biomarkers was compared using chi-square test.
Results:Stage II, III, and IV was 14 (50.0%), 11 (39.3%), and 3 (10.7%) patients, respectively. Hormone receptors-positive tumors were 15 (53.6%) patients and 9 (32.1%) patients received tyrosine kinase inhibitors with or without trastuzumab. Positive expression of initial EGFR, HER-3, HER-4, and IGF-1R was determined in 15 (53.6%), 11 (39.3%), 22 (78.6%), and 14 (50.0%) patients, respectively. Although there was no statistical significance, good responders showed a higher proportion of positive HER-3 expression. Among 23 poor responders, growth factor receptors family expression showed a trend of concordant results, however, unpredictively certain patients demonstrated discordant results between before and after targeted therapy.
Conclusion:Unpredicted correlation of growth factor receptors family expression suggested that complex and personalized resistance mechanisms may be involved to HER-2 directed therapy. However, HER-3 expression might be associated with responsiveness to HER-2 targeted therapy. It would be important to develop diagnostic and therapeutic strategies for overcoming resistance to HER-2 targeted therapy.
INTRODUCTIONApproximately 20% to 30% of invasive breast cancers overexpress or amplify human epidermal growth factor receptor-2 (HER-2) protein or gene [1,2]. Such tumors are associated with poor prognosis and have shown clinical benefits from targeted therapy including trastuzumab in the metastatic or adjuvant setting [3,4]. However, many HER-2 positive breast cancers eventually develop resistance to therapy.
HER family is composed of 4 members: HER-1 which is also known as epidermal growth factor receptor (EGFR), HER-2, HER-3, and HER-4. Although ligand of HER-2 has not been identified yet, heterodimerization of HER-2 with other HER members potently activates intracellular signal transduction pathways including mitogen activated protein kinase pathway and phosphatidylinositol 3-kinase (PI3K) pathway [5]. These signals eventually increase cell proliferation and motility, accelerate angiogenesis, reduce apoptosis, and are associated with tumor invasiveness, metastasis, and survival [6].
Several mechanisms have been proposed in relation with de novo or acquired resistance to HER-2 targeted therapy. These include alteration of binding site of HER-2 to trastuzumab including truncated HER-2, known as p95-HER-2, activation of alternative signaling pathways including insulin-like growth factor-1 receptor (IGF-1R) pathway, change in downstream signaling pathways of HER-2 including upregulation of PI3K pathway, and failure of an appropriate immune-mediated response including low affinity Fc receptor polymorphisms [7-9]. However, development or clinical validation of therapeutic strategies is still remaining to be determined for overcoming resistance to HER-2 targeted therapy. In addition, prognostic or predictive biomarkers including HER-2 are known to be possibly discordant between primary and metastatic breast cancers [10]. However, information on expression of growth factor receptors family other than HER-2, especially before and after administration of therapeutic agents, has been limited in Korean breast cancer patients who received HER-2 targeted therapy.
The aims of this study were to investigate and to compare expression of EGFR, HER-3, HER-4, and IGF-1R before and after HER-2 targeted therapy in primary or metastatic HER-2 positive breast cancer patients.
METHODSPatient selection and clinicopathologic parametersA total of 28 patients having available archive tissue blocks before and after targeted therapy were retrospectively selected from the Severance Hospital of Yonsei University College of Medicine, Seoul, Republic of Korea, between January 2007 and December 2011. HER-2 positive breast cancer patients who had recurrent or metastatic disease without treatment of targeted agents or who diagnosed of disease relapse based on clinico-radiological findings alone without tissue confirm were excluded. All patients were histologically confirmed with HER-2 positive invasive breast cancer and received systemic chemotherapy combined with trastuzumab, tyrosine kinase inhibitors including lapatinib and afatinib, or both.
Five of 28 patients received targeted therapy in the neoadjuvant setting and achieved a pathologic complete response (pCR) after definite surgery without recurrence during follow-up. Therefore, tissue blocks after targeted therapy were not able to be obtained and these 5 patients with a pCR were defined as good responder in this study. Of 28 patients, 23 eventually experienced disease progression or recurrence even though administration of targeted therapy and were defined as poor responder in this study. In 23 patients, a total of 49 formalin-fixed, paraffin-embedded primary or metastatic breast cancer tissue blocks were available and obtained both before and after targeted therapy. HER-2 targeted therapy was administered at the metastatic setting in 6 patients, at the neoadjuvant setting in 4 patients, and at the adjuvant setting in 13 patients. Three tissue blocks were obtained from 3 patients and paired 2 blocks were obtained from 20 patients. Finally, 54 tissue blocks from 28 patients were analyzed.
Clinicopathological information including treatment modalities or expression of hormone receptors (HRs) was obtained from the review of medical records and pathology reports. Tumors with ≥1% nuclear-stained cells by immunohistochemistry were considered positive for estrogen receptor (ER) and progesterone receptor (PR) according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [11]. HER-2 staining was scored as 0, 1+, 2+, or 3+ according to ASCO/CAP guidelines [12]. In cases with a HER-2 2+ result, fluorescence in situ hybridization (FISH) was performed using a PathVysion HER2 DNA Probe Kit (Vysis, Downers Grove, IL, USA) and HER-2 gene amplification was defined as a HER-2 gene/chromosome 17 copy number ratio ≥2.0 according to ASCO/CAP guidelines [12]. HER-2 was considered positive in cases with an immunohistochemistry score of 3+ or gene amplification by FISH. This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea (IRB No. 4-2013-0390). Written informed consent was waived.
Immunohistochemical stainingWith reviewing the archival hematoxylin and eosin stained slides, immunohistochemistry was performed using the whole sections of formalin-fixed, paraffin-embedded tissue blocks. Briefly, 3 µm-thick sections were obtained using a microtome and transferred onto adhesive slides. The whole sections were deparaffinized and rehydrated in usual manner. Antigen retrieval was performed using an electronic pressure cooker for 10 minutes in Triology buffer (Cell Marque Co., Rocklin, CA, USA). After treatment with hydrogen peroxide block solution for 10 minutes, background staining was blocked by incubation in Ultra V Block solution (Thermo Scientific/Lab Vision, Fremont, CA, USA) for 5 minutes at room temperature. After incubation with primary antibodies against EGFR (diluted 1:75, DAK-H1-WT; Dako, Glostrup, Denmark), HER-3 (diluted 1:50, DAK-H3-IC; Dako), HER-4 (diluted 1:50, polyclonal; Thermo Scientific), and IGF-1R (diluted 1:200, polyclonal; Bioss, Woburn, MA, USA), immunodetection was performed using the UltraVision LP detection system (Thermo Scientific/Lab Vision) according to the manufacturer’s instructions. Color was developed with 3,3´-diaminobenzidine and slides were counterstained with Harris hematoxylin. The primary antibody incubation step was omitted in the negative control.
Interpretation of immunohistochemical staining was performed blinded to clinical data of the patients. Expression of EGFR, HER-3, HER-4, and IGF-1R was detected as membrane staining and defined using the following categories in accordance with HER-2 expression scoring system: 0, no immunostaining; 1+, weak or incomplete membrane staining, less than 10% of tumor cells; 2+, complete membranous staining, either uniform or weak in at least 10% of tumor cells; and 3+, uniform intense membranous staining in at least 10% of tumor cells. In this study, arbitrary cutoff was used since no consensus existed for determination of EGFR, HER-3, HER-4, and IGF-1R expression. Staining score of 0 and 1+ was considered negative result while score of 2+ and 3+ was considered positive in this study (Fig. 1).
Statistical analysisThe differences between the discrete variables were evaluated by the chi-square test. Fisher’s exact test was used when appropriate. For the comparison of the means in the case of continuous numerical data, the independent two samples t-test was used. A P-value <0.05 was considered statistically significant. SPSS ver. 20.0 (IBM Inc., Armonk, NY, USA) was used for all statistical analyses.
RESULTSMean age at diagnosis was 46.9 years in 28 patients. Stage II, III, and IV at initial diagnosis of breast cancer was 14 (50.0%), 11 (39.3%), and 3 (10.7%) patients, respectively. ER-positive or PR-positive tumors were 15 (53.6%) patients and HRs-negative tumors were 13 (46.4%) patients. Trastuzumab alone was administered in 19 (67.9%) patients and 9 (32.1%) patients received tyrosine kinase inhibitors with or without trastuzumab. Of 54 tissue blocks, 31 (57.4%) were breast. Locoregional sites were in 9 (16.7%) cases. Lung, brain, bone or soft tissues, liver, and ovary were in 6 (11.1%), 4 (7.4%), 2 (3.7%), 1 (1.9%), and 1 (1.9%) cases, respectively.
Positive expression of initial EGFR, HER-3, HER-4, and IGF-1R was determined in 15 (53.6%), 11 (39.3%), 22 (78.6%) and 14 (50.0%) patients, respectively. Baseline growth factor receptors status, HRs expression and targeted agents were not significantly different between patients with good and poor response to HER-2 targeted therapy (Table 1). Although there was no statistical significance, good responders showed a higher proportion of positive HER-3 expression (P=0.062).
Among 23 poor responders, growth factor receptors family expression was compared between baseline tissue specimens before administration of targeted therapy and resistant tumor tissues after administration of targeted therapy. Table 2 shows significant associations of EGFR expression in all cases but approximately one-fourths patients discordantly changed in EGFR expression. On the basis of subgroup analysis according to expression of HRs, there was no statistically significant association of EGFR expression between initial and recurrent tumors. According to regimens of targeted agents, EGFR showed a trend of concordant results without statistical significance. Comparison of HER-3 expression in all cases was determined no significant associations of HER-3 expression. Similarly to EGFR expression, approximately one-thirds patients discordantly changed in HER-3 expression but a trend of concordant results was noted (Table 3). Interestingly, in subgroups of patients who received trastuzumab alone, HER-3 expression presented a pattern of concordant results but many patients showed negative HER-3 expression. Table 4 shows a trend of concordant HER-4 results without statistical significance in all cases but approximately one-fourths patients showed discordantly changed in HER-4 expression. Similarly, in the analysis of IGF-1R, 8 of 23 (34.8%) patients discordantly changed IGF-1R expression although there was no statistically significant association in all cases (Table 5). By subgroup analysis of HRs-positive tumors, IGF-1R expression showed concordant association with borderline significance between baseline and resistant tumors.
Four blocks from 2 patients were able to check expression of growth factor receptors family before administration of targeted therapy. One patient aged 52-year-old had HRs negative breast cancer with simultaneous lung metastasis at initial diagnosis. EGFR, HER-3, and HER-4 of primary breast cancer and metastatic lung lesion was consistently positive, negative, and positive, respectively. However, IGF-1R of breast and lung lesions was positive and negative, respectively. The other aged 55-year-old had HRs positive tumor. Three years later, lung mass was developed and confirmed as metastatic carcinoma by fluoroscopy-guided lung biopsy. HER-3 alone of breast and lung lesions was consistently negative. EGFR, HER-4, and IGF-1R of primary breast lesion was positive, positive, and negative, respectively. However, EGFR, HER-4, and IGF-1R of metastatic lung lesion was negative, negative, and positive, respectively.
One patient evaluated biomarkers three times. She was 46 year-old and had HRs negative tumor. EGFR, HER-3, HER-4, and IGF-1R of breast cancer was positive, negative, positive, and positive, respectively. After neoadjuvant chemotherapy, she received breast conservation therapy and then adjuvant trastuzumab. Thirteen months later, local recurrent tumor was detected and she underwent salvage mastectomy. EGFR, HER-3, HER-4, and IGF-1R of local recurrent tumor was positive, negative, positive, and negative, respectively. Since then, she received lapatinib. However, lung metastasis occurred 26 months later after initial diagnosis. EGFR, HER-3, HER-4, and IGF-1R of systemic metastatic lesion was positive, positive, negative, and negative, respectively.
DISCUSSIONIn our study, approximately three-fourths of HER-2 positive breast cancers expressed HER-4 and half of cases expressed EGFR and IGF-1R. The lowest proportion of one-thirds was determined in HER-3 expression. Previous studies have shown various positive rates of EGFR, HER-3, HER-4, and IGF-1R expression among HER-2 positive breast cancers, which ranged from 18% to 56%, 9% to 91%, 18% to 59%, and 26% to 44%, respectively [13-18]. Although these wide frequencies of positive growth factor receptors family expression may be partly caused by the different characteristics of study cohorts or the lack of standard methodology among reports, the present study also demonstrated similar results.
Our study evaluated growth factor receptors family using whole sections of biopsy or surgical specimens and compared biomarkers between baseline status before administration of targeted agent and resistant tumor tissues to HER-2 targeted therapy. There were no significant associations of growth factor receptors family expression except EGFR between before and after administration of targeted therapy, which means a certain proportion of patients unpredictively changed in growth factor receptors expression compared to baseline status when recurrent or metastatic tumors were developed. Alternative growth factor receptors family signaling pathways could be involved in resistance to HER-2 targeted therapy [7], however, mechanisms would be very complex and possibly individualized.
Various factors other than HER family and IGF-1R have been implicated in resistance to HER-2 targeted therapy as follows: the HER-2 gene copy number, presence of p95-HER-2, Fc receptor polymorphisms, loss of phosphatase and tensin homolog (PTEN), down-regulation of p27Kip1 expression, activation of mutations of PI3KCA gene, vascular endothelial growth factor receptor, heat shock protein 90 (HSP90), activation of the cytoplasmic tyrosine kinase SRC, and mucin 4 glycoprotein expression [7,8,19,20]. However, recent biomarker analyses in CLEOPATRA trial showed that HER-2 was the only predictive marker for the use of trastuzumab plus pertuzumab-based regimen as first-line treatment in HER-2 positive metastatic breast cancer [21]. HER-2, HER-3 mRNA, and PIK3CA gene mutation were independent prognostic factors by multivariable analyses [21]. HER-3 mRNA was positively associated with favorable prognosis and PIK3CA mutation was negatively related to prognosis.
In our explorative study, higher positive expression of HER-3 was detected in good responders to targeted therapy, with a borderline statistical significance. In addition, by subgroup analysis of patients who treated with trastuzumab alone, 11 of 15 patients (73.3%) who experienced treatment failure showed negative HER-3 expression. Before administration of targeted agent, two patients with lung metastasis checked HER-3 expression in the primary and metastatic lesions and it was negative. These findings suggested that co-expression of HER-2 and HER-3 might be associated with favorable response to targeted therapy. However, further study with larger sample size is necessary to confirm our hypothesis and the prognostic and predictive roles of HER-3 expression in HER-2 positive breast cancers.
Recently, IGF-1R is considered as one promising biomarker for overcoming resistance of HER-2 positive breast cancer. However, previous studies have demonstrated contradictory results regarding a potential role of IGF-1R in breast cancers [17,18,22]. In this study, no definitive roles of IGF-1R expression were shown in HER-2 positive breast cancers, however, in the subgroup analysis of HRs-positive diseases, IGF-1R expression was moderately correlated and might be a potential biomarker for overcoming resistance to HER-2 directed therapy. In a recent study analyzed more than 1,100 patients, IGF-1R expression was frequently expressed in the luminal A/B subtype tumors and positive correlation of IGF-1R with prognosis was demonstrated [18]. Phase II clinical trials of IGF-1R inhibitors are ongoing and these trial results will give a clue regarding the role of IGF-1R and the efficacy and safety of IGF-1R inhibitors in HER-2 positive breast cancer [19].
Major limitation of this study was that sample size was too small to derive definitive conclusions. During study periods, the costs of HER-2 targeted therapy were covered by national health insurance only in selected cases, therefore, a limited number of patients was able to be analyzed in this study. More importantly, immunohistochemical detection or interpretation of growth factor receptors family has not been standardized. Further study is necessary.
In conclusions, many HER-2 positive breast cancers overexpressed EGFR, HER-3, HER-4, or IGF-1R. A certain proportion of growth factor receptors family expression between before and after use of targeted therapy unpredictively showed discordant results, which suggested complex and personalized resistance mechanisms to HER-2 directed therapy. However, HER-3 expression might be associated with responsiveness to HER-2 targeted therapy. In the era of wide use of HER-2 directed therapy for HER-2 positive disease, it would be of importance the development of diagnostic and therapeutic strategies for overcoming resistance to HER-2 targeted therapy.
ACKNOWLEDGMENTSThis study was supported by a faculty research grant of Yonsei University College of Medicine for 2013 (Grant No. 6-2013-0104).
REFERENCES1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82.
2. Park S, Park HS, Koo JS, Yang WI, Kim SI, Park BW. Breast cancers presenting luminal B subtype features show higher discordant human epidermal growth factor receptor 2 results between immunohistochemistry and fluorescence in situ hybridization. Cancer 2012;118:914-23.
3. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92.
4. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84.
5. Sundaresan S, Penuel E, Sliwkowski MX. The biology of human epidermal growth factor receptor 2. Curr Oncol Rep 1999;1:16-22.
6. Sachdev JC, Jahanzeb M. Blockade of the HER family of receptors in the treatment of HER2-positive metastatic breast cancer. Clin Breast Cancer 2012;12:19-29.
7. Thery JC, Spano JP, Azria D, Raymond E, Penault Llorca F. Resistance to human epidermal growth factor receptor type 2-targeted therapies. Eur J Cancer 2014;50:892-901.
8. Orphanos G, Kountourakis P. Targeting the HER2 receptor in metastatic breast cancer. Hematol Oncol Stem Cell Ther 2012;5:127-37.
9. Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther 2011;11:263-75.
10. Chang HJ, Han SW, Oh DY, Im SA, Jeon YK, Park IA, et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J Clin Oncol 2011;41:593-9.
11. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784-95.
12. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118-45.
13. Yonemori K, Tsuta K, Shimizu C, Hatanaka Y, Hirakawa A, Ono M, et al. Immunohistochemical expression of HER1, HER3, and HER4 in HER2-positive breast cancer patients treated with trastuzumab-containing neoadjuvant chemotherapy. J Surg Oncol 2010;101:222-7.
14. Robinson AG, Turbin D, Thomson T, Yorida E, Ellard S, Bajdik C, et al. Molecular predictive factors in patients receiving trastuzumab-based chemotherapy for metastatic disease. Clin Breast Cancer 2006;7:254-61.
15. Smith BL, Chin D, Maltzman W, Crosby K, Hortobagyi GN, Bacus SS. The efficacy of Herceptin therapies is influenced by the expression of other erbB receptors, their ligands and the activation of downstream signalling proteins. Br J Cancer 2004;91:1190-4.
16. Arteaga CL. Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol 2002;29(5 Suppl 14):3-9.
17. Yerushalmi R, Gelmon KA, Leung S, Gao D, Cheang M, Pollak M, et al. Insulin-like growth factor receptor (IGF-1R) in breast cancer subtypes. Breast Cancer Res Treat 2012;132:131-42.
18. Shin SJ, Gong G, Lee HJ, Kang J, Bae YK, Lee A, et al. Positive expression of insulin-like growth factor-1 receptor is associated with a positive hormone receptor status and a favorable prognosis in breast cancer. J Breast Cancer 2014;17:113-20.
19. Mohd Sharial MS, Crown J, Hennessy BT. Overcoming resistance and restoring sensitivity to HER2-targeted therapies in breast cancer. Ann Oncol 2012;23:3007-16.
20. Mukohara T. Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci 2011;102:1-8.
Fig. 1.Immunohistochemical expression of EGFR, HER-3, HER-4, and IGF-1R in HER-2 positive breast cancer tissues (immunohistochemical staining, ×100). (A) Negative and (B) positive results of EGFR, (C) negative and (D) positive results of HER-3, (E) negative and (F) positive results of HER-4, and (G) negative and (H) positive results of IGF-1R. EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; IGF-1R, insulin-like growth factor-1 receptor. 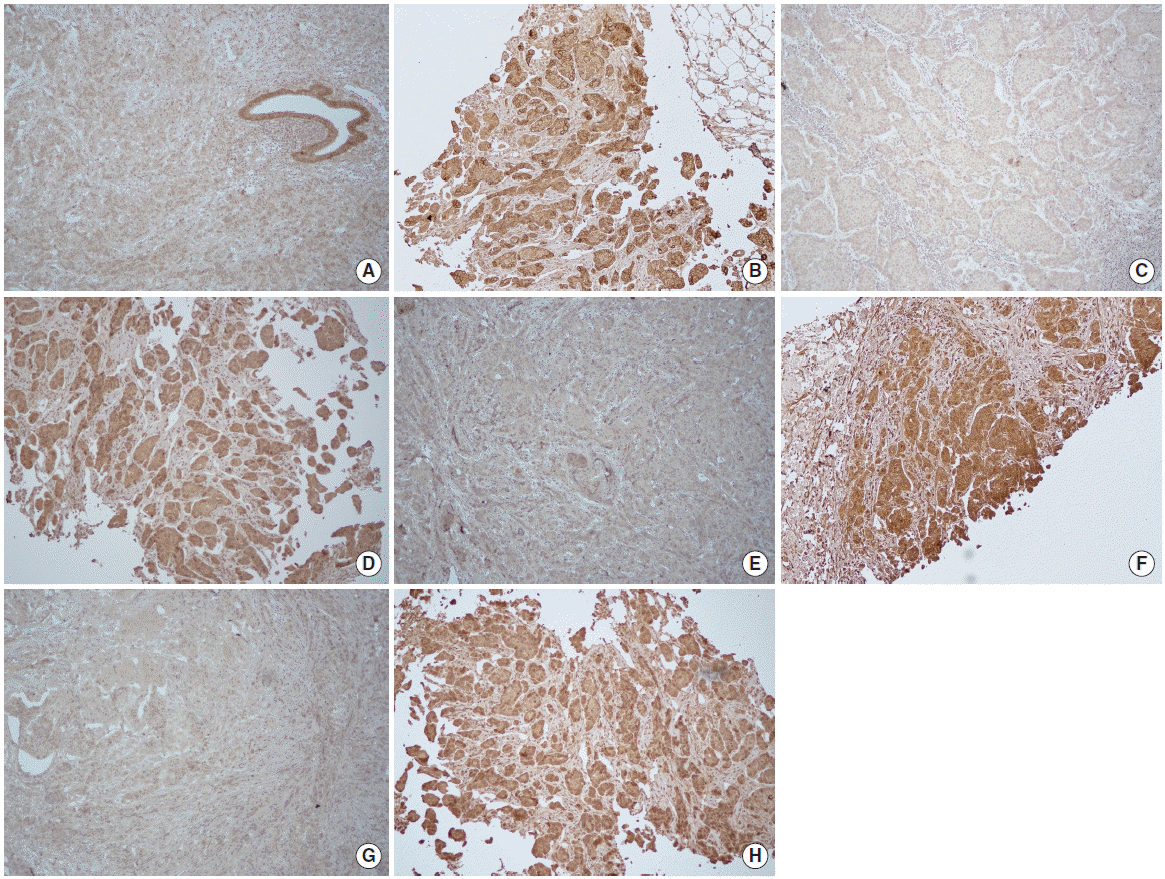
Table 1.Comparison of initial growth factor receptors and clinicopathological parameters between patients with good and poor response to targeted therapy
Table 2.Comparison of EGFR before and after targeted therapy
Table 3.Comparison of HER-3 before and after targeted therapy
Table 4.Comparison of HER-4 before and after targeted therapy
Table 5.Comparison of IGF-1R before and after targeted therapy
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||