ABSTRACTPurpose:The prognosis of patients with liver metastasis from gastric cancer is poor and the optimal treatment remains undetermined. This study identified prognostic factors for survival of patients with metachronous liver metastasis with no other metastatic site after gastrectomy for primary gastric cancer. We also evaluated the clinical impact of hepatic resection.
Methods:Between 1997 and 2013, 19,588 curative gastrectomies for gastric adenocarcinoma were performed and 52 patients were diagnosed with metastasis to only the liver. We retrospectively analyzed the clinicopathologic factors of these patients.
Results:The median time from gastrectomy to diagnosis of liver metastasis was 16 months (range, 1–65 months). Median survival time after the diagnosis of liver metastasis was 13 months (range, 3–64 months). The 1-year, 2-year, and 3-year patient survival rates after diagnosis of liver metastasis were 53.8%, 26.6%, and 19.9%, respectively. Twelve patients (23%) underwent liver resection for liver metastasis. The 1-year, 3-year, and 5-year overall survival rates were 92%, 42%, and 42% in the hepatic resection group and 43%, 13%, and 7% in the non-hepatic resection group (P=0.002). Multivariate analysis showed that hepatic resection, pathologic stage Ⅰ and Ⅱ of the primary tumor, and intestinal type in Lauren classification were predisposing factors for patient survival.
INTRODUCTIONA diagnosis of distant metastases of gastric cancer is correlated with very poor prognosis and the clinicopathologic characteristics of long-term gastric cancer survivors are not well known. Liver metastasis occurs in 3.5% to 14% of patients who undergo surgery for gastric cancer [1-4]. Many patients with liver metastasis are not suitable candidates for hepatic resection, and surgical resection of liver metastasis from gastric cancer is rarely indicated.
Chemotherapy is regarded the standard treatment for recurrent and metastatic gastric cancer because of multiple lobe metastases, peritoneal dissemination, and extensive lymph node metastases, or direct invasion of other organs [5]. However, the reported median survival after chemotherapy for patients with unresectable and metastatic gastric cancer is 11.0 to 13.8 months [6-8]. These results are unsatisfactory in light of recent advances in chemotherapy for colorectal cancer [9]. Thus, the management of liver metastasis is difficult and many patients with liver metastasis are not suitable candidates for hepatic resection.
The efficacy of operative resection for hepatic metastases from colorectal cancer is established, with at 5-year survival rates of 40% to 50% and 10-year survival rates of 20% to 30% after hepatectomy [9-11]. Indications for hepatic resection have therefore been extended to technically resectable colorectal cancer with liver metastases. However, the clinical effects of hepatic resection for metastases from gastric cancer remain controversial [12], although some studies indicate that hepatic resection is associated with long-term survival [1,3,4,13,14]. Most previous reports evaluated the benefits of hepatic resection on survival only among patients who underwent hepatic resection. However, all studies included patients with synchronous and metachronous metastases.
In this study, we retrospectively identified prognostic factors for survival in patients with metachronous liver metastasis with no other metastatic site after gastrectomy for gastric cancer. We also compared clinicopathologic characteristics of patients with and without curative hepatic resection.
METHODSPatientsFrom January 1997 to July 2013, 19,588 patients underwent curative gastric resection with D2 lymph node dissection for gastric adenocarcinoma at Samsung Medical Center, Seoul, Korea. No gross residual disease was evident at the time of operation. After gastrectomy, 52 patients were diagnosed with metachronous liver metastasis and no other metastases. Data on baseline demographics, treatment methods, and clinicopathologic findings of the primary gastric cancer and liver metastases were retrospectively obtained from electronic medical records of patients. Clinicopathologic factors were analyzed by comparing patients subdivided by age; gender; tumor location; tumor size; length of proximal and distal resection margin; T stage; lymph node metastases; pathologic stage; histologic differentiation; Lauren classification of primary tumor; number, distribution, and size of liver metastasis; and treatment by hepatic resection, chemotherapy, or radio frequency ablation. Pathologic stage and tumor, node, metastasis (TNM) stage were classified according to the American Joint Committee on Cancer (AJCC) seventh edition. Hepatic resection for gastric cancer metastases was performed only when curative resection was intended. Exclusion criteria were presence of extrahepatic distant metastases such as peritoneal seeding or presence of another malignancy.
Surveillance after curative gastrectomyAll patients were observed at 3-month intervals during the first year and every 6 months thereafter for 5 years after curative gastrectomy. Examinations included endoscopy and computed tomography(CT). Patients with a suspected lesion by CT underwent additional imaging including magnetic resonance imaging, ultrasonography, and/or positron emission tomography. Overall survival time was measured from the date of detection of hepatic metastasis to the date of death from any cause or the last follow up.
Indications for liver resectionCurative hepatic resection was defined as macroscopically and microscopically undetectable tumors. Indications for resection of hepatic metastases included: 1) no signs of peritoneal dissemination or any other distant metastases on preoperative imaging; 2) feasibility of complete tumor resection including multiple hepatic metastases(unilobar or bilobar); 3) good performance status of patients; and 4) acceptable hepatic function based on serum liver function test panel, Child-Pugh score, and indocyanine green test. Preoperative assessment of indications for resection was performed in a multidisciplinary meeting. Exclusion criteria for hepatic resection were unresectable multiple bilobar metastases or more than four metastatic lesions.
Statistical analysisCategorical variables were expressed as percentages and compared using a χ2 test or Fisher’s exact test. Continuous variables were expressed as median and range and compared using the Mann-Whitney U test. Data on patients who were alive or lost to follow-up were censored. The arbitrary cut-off value for each continuous variable was determined using receiver operating characteristics curves. Overall survival was calculated using the Kaplan-Meier method. Predictors of overall survival in univariate analysis were determined using the Kaplan-Meier method and log-rank test. Clinical and pathologic variables with prognostic significance (P<0.20) in univariate analysis were entered into a Cox multivariate proportional hazards model to determine factors independently predictive of overall survival. P<0.05 was considered significant. Analysis was carried out using SPSS ver. 21.0 (IBM Co., Chicago, IL, USA).
RESULTSBaseline characteristicsPatient demographics at time of curative gastrectomy are presented in Table 1. Median follow-up duration after curative gastrectomy was 25 months (range, 7-130 months). Median time from time of gastrectomy to diagnosis of liver metastasis was 16 months (range, 1-65 months). Clinicopathologic characteristics of the patients are presented in Table 2. Major hepatectomy (resection of more than three segments) was performed in five patients (right hemihepatectomy in four and left hemihepatectomy in one) and minor hepatectomy was performed in seven patients (right posterior sectionectomy in one, left lateral sectionectomy in three, and tumorectomy in three). In solitary liver metastasis (n=22), hepatic resection (n=7), radiofre-quency ablation (n=1), and chemotherapy (n=10) were performed as initial treatment. However, four patients refused treatments. No mortality occurred within 30 days after surgery. Chemotherapy was given to 43 patients, and three also received radiofrequency ablation among these patients. At the last follow-up, eight patients were alive with a median survival time after diagnosis of liver metastasis of 13 months (range, 3-64 months). The 1-year, 2-year, and 3-year patient survival rates after diagnosis of liver metastasis were 53.8%, 26.6%, and 19.9%, respectively (Fig. 1).
Risk factors for patient survivalUnivariate analyses revealed that the following factors were positively associated with patient survival after diagnosis of liver metastasis: a distal resection margin greater than 3 cm of the resected stomach, pathologic stage Ⅰ and Ⅱ of the primary tumor, intestinal type in Lauren’s classification of primary tumor, and hepatic resection of liver metastasis (Table 3). Multivariate analysis using the Cox regression analysis showed that hepatic resection (odds ratio [OR], 3.03; 95% confidence interval [CI], 1.23–7.45; P=0.016), primary tumor pathologic stage (OR, 2.71; 95% CI, 1.27–5.79; P=0.010), and intestinal type in Lauren classification (OR, 2.81; 95% CI, 1.40–5.65; P=0.004) were predictors that significantly affected prognosis for all patients(Table 4). The influence of hepatic resection, pathologic staging of primary tumor, and Lauren classification are shown in Fig. 2.
Comparison between hepatic resection and non-hepatic resectionNo significant differences were observed between patients with and without hepatic resection for age, gender, primary tumor location, primary tumor size, resection margin, T staging, lymph node metastasis, differentiation, tumor number of liver metastasis, and tumor size of liver metastasis (Table 5). Hepatic resection was not performed for the following reasons: unresectable bilobar metastases (n=19), chemotherapy as the initial treatment of liver metastases (n=14), and refusal of liver resection (n=7). The proportions of pathologic stage III primary tumors, total gastrectomy, and bilobar liver metastasis were higher in the non-hepatic resection group than in the hepatic resection group. Overall survival rates for the hepatic resection group were 92% for 1 year, and 42% for both 3 and 5 years. Overall survival rates for the non-hepatic resection group were 43% for 1 year, 13% for 3 years, and 7% for 5 years (P=0.002). In the process of determining hepatic resection, general condition of the patient or state of liver can be affected.
American Society of Anesthesiologist (ASA) score and Child-Pugh score were investigated at the time of diagnosis of hepatic metastasis. There was no significant difference between the hepatic resection group and the non-hepatic resection group (Table 5).
DISCUSSIONThe present study demonstrated that favorable outcomes for patients with metachronous liver metastasis who received a D2 lymphadenectomy at gastrectomy with hepatic resection were associated with pathologic stage Ⅰ and Ⅱ and intestinal type of Lauren’s classification for the primary tumor.
In Lauren’s classification, the intestinal type is characterized by cohesive cells which form gland-like structures, while the diffuse type is characterized by non-cohesive and scattered tumor cells which lack cell-to-cell interactions and infiltrate the stroma as single cell or small subgroups [15]. The prognostic relevance of Lauren’s classification is still controversial. Several studies have indicated that patients with intestinal-type tumors had a better outcome than those with diffuse-type tumors because of the difference of molecular characteristics [16,17]. One study reported that the higher percentage of patients with advanced lymph node metastases and advanced tumor invasion in the diffuse-type gastric carcinoma as compared with the intestinal type may contribute to the poor prognosis of patients [18]. Other study also showed that the more dismal prognosis of diffuse-type gastric carcinoma than intestinal-type could be explained by propensity of deeper invasion and emerging peritoneal cancer cell [16]. These studies may contribute to the poor prognosis of patients with diffuse-type. The precise mechanism by which gastric adenocarcinoma pathologic staging I and II and intestinal type of Lauren’s classification improves the survival of patients with hepatic metastasis remains unclear, but we propose that primary gastric adenocarcinoma with pathologic stage III and diffuse type of Lauren’s classification might have already acquired metastatic potential for other organs
The use of liver resection in metachronous liver metastases maybe considered in resectable tumor, but only highly selected patients are eligible for liver resection. The majority of patients with unresectable bilobar metastases were not treated with surgery, therefore unresectable bilobar tumor may profound affect multivariate analysis for patient survival. However, this finding may contribute to early detection of recurrent disease because liver resection is feasible and most effect.
In this study, median overall survival was 27.2 months for the hepatic resection group and 9.8 months for the non-hepatic resection group. Overall 1-year, 3-year, and 5-year survival rates were 92%, 42%, and 42% for the hepatic resection group compared with 43%, 13%, and 7% for the non-hepatic resection group (P=0.002).
In general, one-fifth of all patients with liver metastasis can undergo hepatic resection [4,19-22]. In this study, the proportion of patients undergoing hepatic resection was 23%, similar to that in other studies. Previous studies reported unsatisfactory results, with a 5-year survival rate between 11% and 26% after liver resection [14,19,22], and two-thirds of patients developing intrahepatic recurrence [21]. However, most of these studies included synchronous and/or metachronous liver metastasis. Metachronous metastasis after curative gastrectomy typically has a favorable outcome [4,19]. In our study, the 5-year survival rate of the hepatic resection group was 42%, most likely because we included only patients with metachronous liver metastasis.
Our study revealed that liver resection is not an absolute surgical indication for gastric cancer liver metastasis. Nonetheless, non-surgical treatment might not influence the long-term survival for patients with this condition. Newly developed chemotherapeutic agents have changed the role of surgical resection in the treatment of liver metastasis of colorectal cancer, and liver resection is now an important tool in multidisciplinary treatments and a standard treatment for colorectal liver metastases [9]. Our data revealed that liver resection might allow long-term survival for selected patients with hepatic metastases from gastric cancer.
This study had several limitations. The first is the selective bias in choosing patients for liver resection. The second is the small number of patients and retrospective analysis of data from a single center. Large, controlled multicenter studies or a detailed meta-analysis will be necessary to select patients who have the greatest benefit from surgical treatment of metachronous gastric cancer liver metastasis.
In conclusion, this study indicated that liver resection, pathologic stage Ⅰ or Ⅱ of primary tumor and intestinal type by Lauren classification were favorable factors for survival of patients with metachronous liver metastasis of gastric cancer. Therefore, hepatic resection might be indicated for patients with these clinical features.
REFERENCES1. Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery 2003;133:507-11.
2. Marrelli D, Roviello F, De Stefano A, Fotia G, Giliberto C, Garosi L, et al. Risk factors for liver metastases after curative surgical procedures for gastric cancer: a prospective study of 208 patients treated with surgical resection. J Am Coll Surg 2004;198:51-8.
3. Zacherl J, Zacherl M, Scheuba C, Steininger R, Wenzl E, Muhlbacher F, et al. Analysis of hepatic resection of metastasis originating from gastric adenocarcinoma. J Gastrointest Surg 2002;6:682-9.
4. Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg 2002;235:86-91.
5. Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today 2010;40:287-94.
6. Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063-9.
7. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97.
8. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21.
9. Frankel TL, D’Angelica MI. Hepatic resection for colorectal metastases. J Surg Oncol 2014;109:2-7.
10. Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 2007;11:1057-77.
11. Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6.
12. Lordick F. To resect or not resect in metastatic gastric cancer: that is the question! Gastric Cancer 2012;15:229-31.
13. Roh HR, Suh KS, Lee HJ, Yang HK, Choe KJ, Lee KU. Outcome of hepatic resection for metastatic gastric cancer. Am Surg 2005;71:95-9.
14. Shirabe K, Shimada M, Matsumata T, Higashi H, Yakeishi Y, Wakiyama S, et al. Analysis of the prognostic factors for liver metastasis of gastric cancer after hepatic resection: a multi-institutional study of the indications for resection. Hepatogastroenterology 2003;50:1560-3.
15. Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol 2014;20:5679-84.
16. Yamashita K, Sakuramoto S, Katada N, Futawatari N, Moriya H, Hirai K, et al. Diffuse type advanced gastric cancer showing dismal prognosis is characterized by deeper invasion and emerging peritoneal cancer cell: the latest comparative study to intestinal advanced gastric cancer. Hepatogastroenterology 2009;56:276-81.
17. Miyahara R, Niwa Y, Matsuura T, Maeda O, Ando T, Ohmiya N, et al. Prevalence and prognosis of gastric cancer detected by screening in a large Japanese population: data from a single institute over 30 years. J Gastroenterol Hepatol 2007;22:1435-42.
18. Qiu MZ, Cai MY, Zhang DS, Wang ZQ, Wang DS, Li YH, et al. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med 2013;11:58.
19. Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Yoshidome H, et al. Benefits and limits of hepatic resection for gastric metastases. Am J Surg 2001;181:279-83.
20. Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, et al. Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol 2007;37:836-42.
Figure 1.Patient survival after liver metastasis. Survival rates after diagnosis of metachronous liver metastasis were 53.8% for 1 year, 26.6% for 2 years, and 19.9% for 3 years. 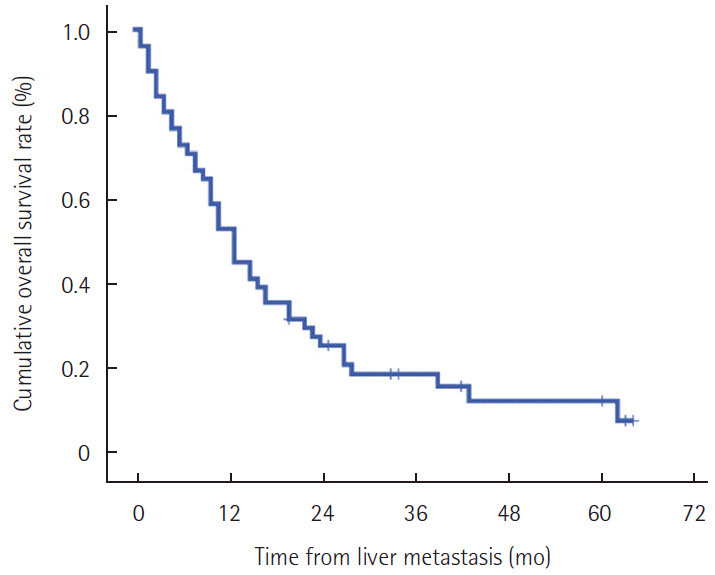
Figure 2.Overall survival after liver metastasis with respect to (A) pathologic staging of primary tumor, (B) intestinal type in Lauren classification, and (C) effect of hepatic resection. 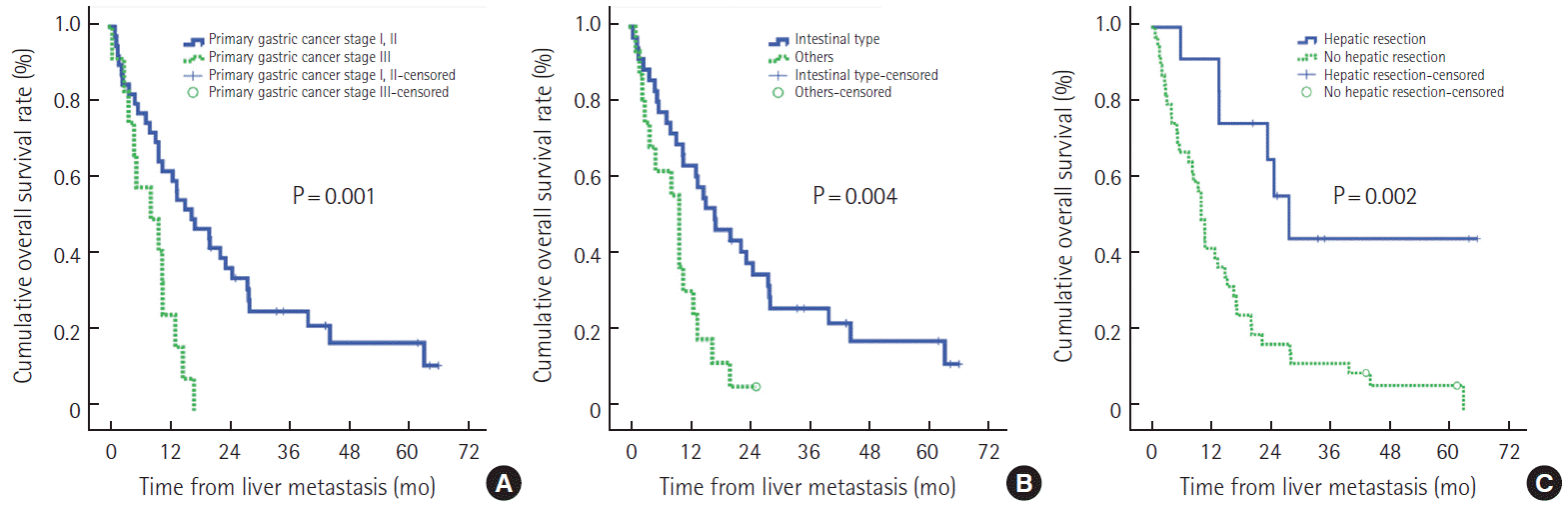
Table 1.Patient characteristics at time of curative gastrectomy
Table 2.Clinicopathologic characteristics of patients with metachronous liver metastasis Table 3.Univariate analysis of patient survival
Table 4.Multivariate analysis for patient survival
Table 5.Comparison between patients with and without hepatic resection
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||