Pancreatic metastasis from papillary thyroid cancer: a case report and literature review
Article information
Abstract
Pancreatic metastasis from papillary thyroid cancer (PTC) is extremely rare; only 18 cases have been reported in the literature. However, several reviews have highlighted similar characteristics between metastatic and primary pancreatic tumors. The patient was a 51-year-old male with a history of total thyroidectomy, modified radical neck dissection, and radioactive iodine ablation for PTC in 2014. Nodules suspected of metastasis were found in both lungs on chest computed tomography (CT). However, after 6 months, a follow-up chest CT showed no increase in size; thus, a follow-up observation was planned. Six years after his initial diagnosis, abdominal CT and pancreas magnetic resonance imaging revealed a 4.7 cm cystic mass with a 2.5 cm enhancing mural nodule in the pancreas tail. We diagnosed the pancreatic lesion as either metastatic cancer or primary pancreas cancer. The patient underwent distal pancreato-splenectomy. After surgery, the pathological report revealed that the mass was metastatic PTC. Pancreatic metastasis from PTC indicates an advanced tumor stage and poor prognosis. However, pancreatectomy can increase the survival rate when the lesion is completely resectable. Therefore, surgical resection should be considered as a treatment for pancreatic metastasis from PTC.
INTRODUCTION
Papillary thyroid cancer (PTC) is the most common type of well-differentiated endocrine malignancy [1]. PTC has an excellent prognosis with a 5-year disease-specific survival rate of 98%. The main route of metastasis for PTC is local spread to the lymph nodes of the neck [1]. Around 5% of patients have systemic metastases, most commonly to the lungs and bones [1]. PTC metastasis to the pancreas is extremely rare. To date, only 18 cases have been reported in the literature [2–11]. According to the literature, diagnosis is difficult due to the occurrence of pancreatic metastasis after a long period and lack of specific imaging findings and clinical standards. Some reports have revealed similar characteristics between pancreatic metastatic tumors and primary pancreatic tumors. Here, we report a patient who underwent surgical resection for pancreatic metastasis detected 6 years after the first PTC diagnosis. Informed consent was waived because the study had a retrospective nature and the analysis used anonymous clinical data.
CASE REPORT
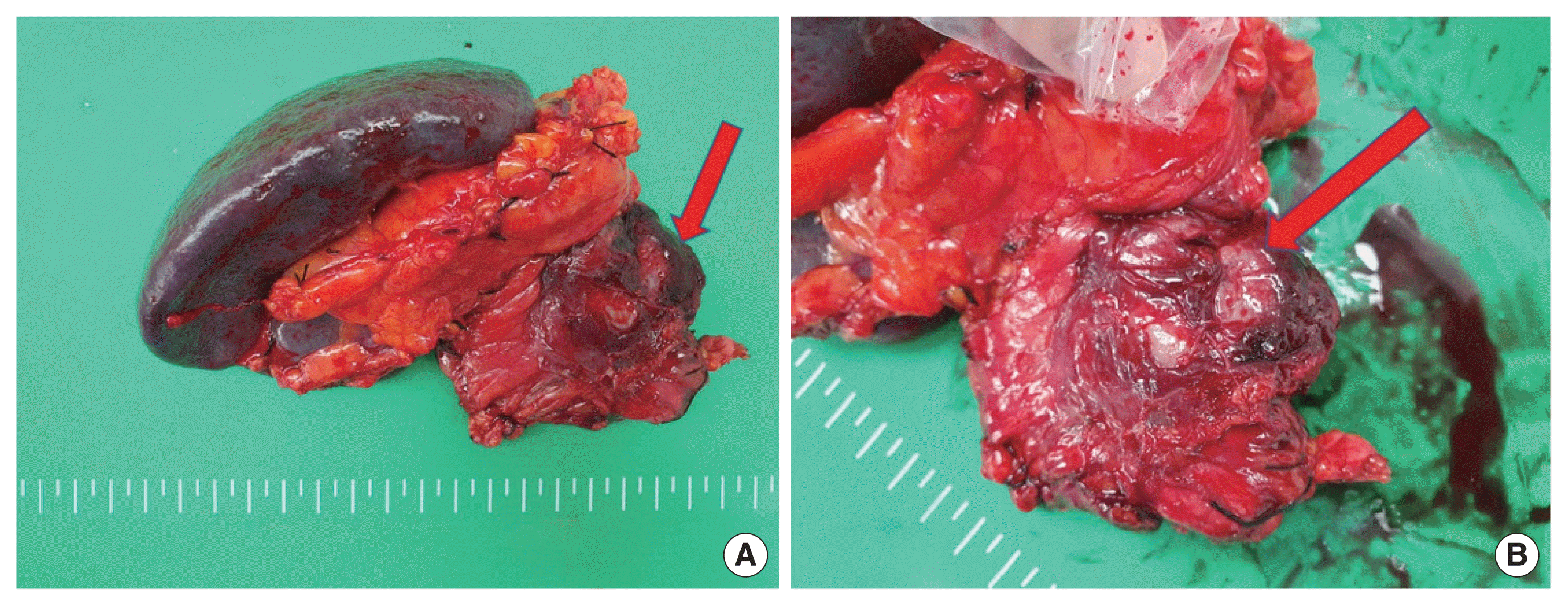
The patient was a 51-year-old male with a history of total thyroidectomy and modified radical neck dissection for PTC in 2014. According to the surgical findings, a tumor lesion was found to invade the trachea, and tracheal shaving was additionally performed. Postoperative pathological diagnosis indicated PTC (TNM classification: pT3N1b) requiring radioactive iodine (RAI) ablation. Three months after RAI ablation, a recurrent mass was found in the trachea on imaging study, and tracheal mass resection was performed. Nodules suspected of metastasis were found in both lungs on chest computed tomography (CT). However, the follow-up chest CT examination after 6 months did not detect an increase in size, and follow-up of the patient was decided. Six years after the initial diagnosis, the patient was referred for surgery for a pancreatic tail mass discovered incidentally on an abdominal CT performed to diagnose the cause of uncontrolled diabetes. All laboratory findings were within normal limits except for a high glucose level (485 mg/dL). The levels of tumor markers were high, with a carbohydrate antigen 19-9 level of 110.8 U/mL (normal range, 0–39 U/mL) and a carcinoembryonic antigen level of 10.54 ng/mL (normal range, 0–5 ng/mL). A high thyroglobulin level of 350 ng/mL (normal range, 1.4–78 ng/mL) was also detected. Abdominal CT showed a 4.7 cm cystic mass with an enhancing mural nodule in the pancreatic tail, rule out malignant cystic neoplasm (Fig. 1). Moreover, pancreas magnetic resonance imaging (MRI) showed pancreas tail with a well-circumscribed dominant cystic mass of 5.6×5.0 cm with a 2.6 cm, mural-enhancing solid portion on the posterior wall with diffusion restriction (Fig. 2). The positron emission tomography-CT (PET-CT) scan showed multiple tiny to small nodules without significant hypermetabolism in both lungs, with a slight size increase or with newly developed lesions, and a 5.8 cm cystic mass in the pancreatic tail, with somewhat focal hypermetabolism in the solid portion (SUV 2.5) (Fig. 3). Based on the imaging findings, we diagnosed the pancreatic lesion as either metastatic cancer or primary pancreatic cancer, and we planned for distal pancreato-splenectomy (DPS). During surgery, the pancreatic tail mass and mesocolon were abutting, therefore, DPS including local excision of mesocolon was performed. Following resection, gross histology of the pancreatic tail mass showed small hematomas and necrotic lesions in the cystic mass. A 2.5 cm-sized solid portion was detected in the cystic mass (Fig. 4). Immunohistochemical analysis showed that the solid portion was positive for CK7, TTF-1, thyroglobulin, and BRAF V600E (3+). The solid portion of the pancreatic tail cystic mass was confirmed as metastatic PTC based on histopathological findings. The patient had an uneventful recovery after the operation and abdomen and chest CT was performed at the scheduled follow-up (at 6-month intervals) after surgery. The nodules suspected of metastasis in both lungs showed non-specific interval changes on serial follow-up chest CT, and there was no recurrence or metastasis of the tumor 3 years after the pancreatic surgery. Abdominal CT was performed to confirm tumor recurrence and metastasis. In addition, chest CT was used to check whether the size of the mass had increased, and an increase in thyroglobulin level was observed. If these findings occur, chemotherapy with a tyrosine kinase inhibitor (sorafenib) can be planned.

Abdominal computed tomography showed a 4.7-cm-sized pancreatic tail cystic mass and a 2.3-cm-sized enhancing mural nodule in the cystic mass lumen.

Abdominal magnetic resonance imaging (enhance) showed a 2.3-cm-sized enhancing mural nodule (arrow in A, T1WI) with a 5.6-cm-sized well-circumscribed cystic mass in the pancreatic tail (arrow in B, T2WI).
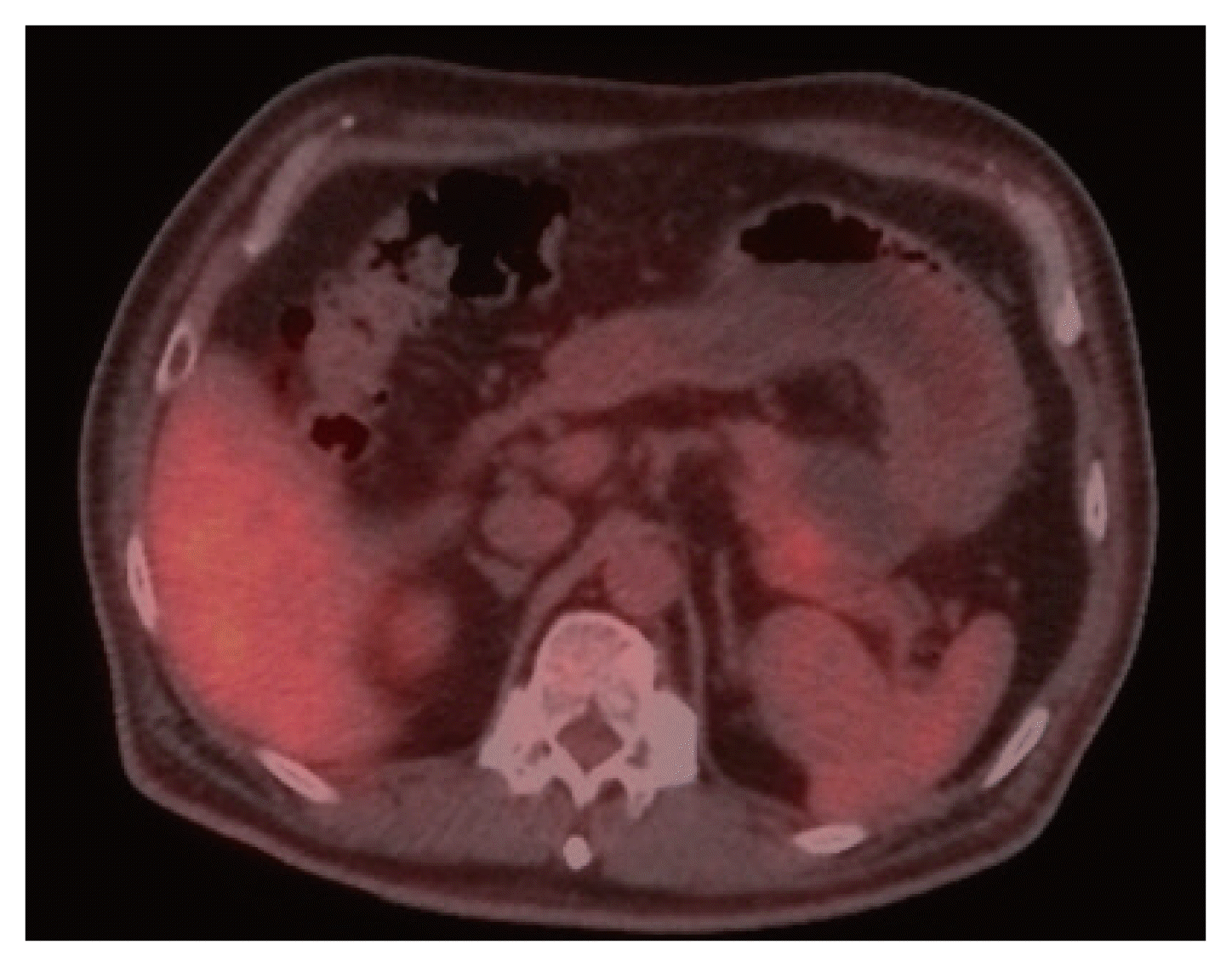
Positron emission tomography-computed tomography showed a 5.8-cm-sized cystic mass in pancreatic tail, with somewhat focal hypermetabolism in solid portion (SUV 2.5).
DISCUSSION
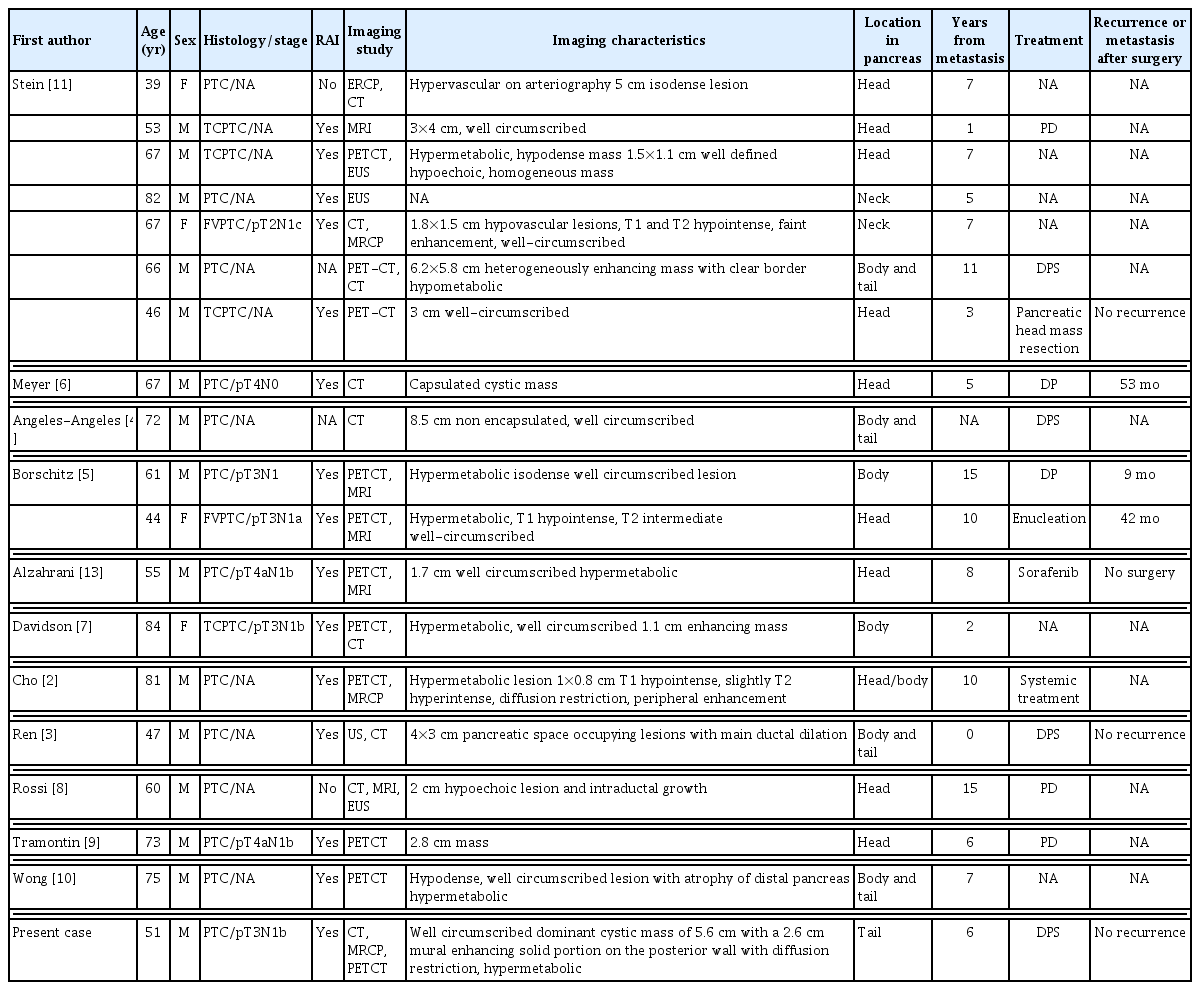
PTC is considered as a low-grade malignancy. Metastases are most often found in the cervical lymph nodes. Distant metastases are uncommon and usually occur in the bones, lungs, and thoracic lymph nodes [1]. According to the literature, metastasis of PTC to the pancreas is extremely rare. Only 18 cases have been reported thus far (Table 1). Patients can be diagnosed with pancreatic metastasis from PTC at various ages (mean age, 59 years), and it occurs mostly in males (male 15 cases and female 4 cases). Most patients are diagnosed with PTC at the initial diagnosis [2–5, 8–10] (tall cell PTC 4 cases [7,11] and follicular variant PTC 2 cases [5]) and undergo RAI ablation [2,3,5,6,9,10]. It has been observed that it takes an average of 7 years for pancreatic metastasis to occur. In our case, involving a 51-year-old male patient, similar to the previously reported literature, the patient was initially diagnosed with PTC (TNM classification: pT3N1b), and after 6 years, pancreatic metastasis was incidentally detected, further MRI and PET-CT were performed.
According to the literature, diagnosis is difficult due to the occurrence of pancreatic metastasis after a long period and lack of specific imaging findings and clinical standards. In some studies, the characteristics of pancreatic metastatic tumors are similar to those of primary pancreatic tumors. Metastatic tumors clinically mimic primary pancreatic tumors and have been reported to appear as a solitary pancreatic mass in imaging studies. Based on CT findings, metastatic tumors typically show a well-circumscribed lesion pattern with contrast enhancement. In addition, endoscopic ultrasound (EUS) usually reveals a hypoechoic hypodense pattern, and these characteristics are similar to those of primary pancreatic tumors [4,11,12]. Therefore, it is necessary to determine whether the pancreatic lesion is a primary or metastatic tumor.
EUS-guided biopsy may be an appropriate diagnostic method, which has a sensitivity of 80% to 90%, a specificity of nearly 100%, and an accuracy in diagnosing metastatic lesions of 89% [8,12]. However, in our case, we observed a cystic mass with an enhancing mural nodule on imaging, mimicking a cystic pancreatic tumor. Therefore, EUS-guided biopsy was not performed, and surgical resection was performed under a diagnosis of a primary or metastatic pancreatic tumor based on imaging findings.
A review of the literature found that surgery was performed in 10 out of 18 cases of pancreatic metastasis from PTC, indicating that the surgical method differed depending on the tumor’s location. For example, pancreaticoduodenectomy (PD) [8,9] or enucleation [5,11] was performed when the tumor was located in the pancreatic head, and distal pancreatectomy (DP) [5,6] or DPS [3,4] was performed when the tumor was located in the body or tail. In our case, DPS was performed because the tumor’s location was in the tail.
In the cases reported by Stein et al. [11], Rossi et al. [8], and Tramontin et al. [9], metastasis to the pancreatic head was found at an average of 7.3 years after the first diagnosis. PD was performed as a treatment, and no recurrence or metastasis to other organs after surgery was reported in all of them. In 2010, Borschitz et al. [5] reported the presence of a tumor in the pancreatic head. Excision was performed for pancreatic metastasis from PTC, and lymph node metastasis occurred 42 months after surgery. When DP (Borschitz et al. [5], Meyer and Behrend [6]) or DPS (Ren et al. [3], Angeles-Angeles et al. [4], and Stein et al. [11]) was performed because the tumor was located in the body or tail, metastasis was found at an average of 9.2 years after the first diagnosis. Among these cases, multiple metastases were found after surgery in two cases. Meyer and Behrend [6] reported a tumor that recurred with death 53 months after surgery, and Angeles-Angeles et al. [4] reported a tumor that recurred 9 months after surgery with death 3 years later. In two cases, there was no recurrence after surgery for pancreatic metastasis from PTC (Ren et al. [3] and Stein et al. [11]). In our case, metastasis to the pancreatic tail was found 6 years after the first diagnosis, DPS was performed on this lesion, and no recurrence or metastasis was found until 3 years after surgery.
Two cases were reported in which no surgical treatment was performed for pancreatic metastasis from PTC. Alzahrani et al. [13] reported a case of sorafenib administration instead of surgical treatment for a PTC patient with pancreatic metastasis. In the case reported by Cho et al. [2], the patient was 81 years old, and systemic chemotherapy was performed instead of surgical treatment because of multiple organ metastases.
Whether surgery for metastatic pancreatic tumors benefits patients’ prognosis is controversial because of the high morbidity and mortality after pancreatectomy. However, when the metastatic pancreatic lesion is a single metastatic tumor, pancreatectomy can increase the 5-year survival rate up to 31% [14]. Therefore, pancreatectomy may be beneficial for treating a patient without multiple organ metastases [6]. In 2006, Reddy and Wolfgang [15] reported the following patient selection criteria: primary cancer type associated with successful outcomes, control of the primary cancer site, isolated metastases, resectability of the metastasis, and patient fitness to tolerate pancreatectomy [15].
In summary, pancreatic metastasis from PTC is extremely rare, which indicates the advanced stage of the tumor, and the prognosis is very poor. However, pancreatectomy can increase the survival rate when the metastatic lesion is completely resectable. Therefore, surgical resection should be considered as a treatment for pancreatic metastasis from PTC.
Notes
No potential conflict of interest relevant to this article was reported.
FUNDING
None.