Utility of sentinel lymph node biopsy in papillary thyroid microcarcinoma
Article information
Abstract
Purpose
There are many studies on sentinel lymph node (SLN) biopsy in thyroid carcinoma but SLN biopsy (SLNB) in papillary thyroid carcinoma (PTC) remains open to debate. Therefore in this retrospective study, the usefulness of SLNB in thyroid carcinoma patients who had micro-PTC without cervical lymphadenopathy was assessed.
Methods
SLNB was performed in 114 patients who were diagnosed with micro-PTC in a single lobe without palpable or ultrasound-detected lymph node at the tertiary center between January 2012 and December 2013. After SLNB, all patients underwent total thyroidectomy and central neck dissection or thyroid lobectomy and central neck dissection of the single side.
Results
SLNs were identified in 112 of 114 patients with 41 positive SLNs and 71 negative SLNs on intraoperative frozen sections. However, eight negative patients were found to be positive in the final pathology. Sentinel node identification rate and false negative value of SLNB were 98.2% and 11.3%, respectively. In the univariate analysis, higher lymph node metastasis was detected in men than in women. Higher detection number of SLN showed higher probability of lymph node metastasis.
Conclusion
SLNB may be helpful in papillary thyroid cancer, especially in male patients. Also, it is useful for the staging of nodal status and clearance of persistent disease.
INTRODUCTION
With the development of ultrasound, the annual incidence of thyroid carcinoma especially microcarcinoma has been increasingly detected [1]. Papillary thyroid carcinoma (PTC) accounts for about 85% of thyroid malignancy. Lymph node metastasis is found in about 35% of PTC patients and is reported to be an independent factor affecting the recurrence of PTC [2].
However, the recent American Thyroid Association (ATA) guidelines do not recommend the dissection of prophylactic central compartment neck in T1–2 PTC patients with no clinical evidence of lymph node metastasis [3] and hence histologic N staging is not possible in these patients. Unfortunately, lymph node metastasis could exist in small size PTC [4], thus N staging is necessary for all PTC patients.
Preoperative ultrasound identifies noticeable cervical lymph node in 20% to 30% of patients [5]. Lymph node metastasis affects the recurrence of PTC but under the new ATA guidelines, the exact nodal stage of T1–2 PTC patients is not evaluated.
Sentinel lymph nodes (SLNs) first drain the lymph of primary cancer in local tissues and biopsy can accurately predict the state of regional lymph nodes. SLN biopsy (SLNB) is widely accepted as the standard procedure for treatment and correct nodal staging for lymph node dissection (LND) in patients with breast cancer [6] and malignant melanoma. If metastasis is detected, LND will be performed and if metastasis is not detected, unnecessary treatment can be avoided to reduce surgical complications [6,7]. SLNB in PTC remains open to debate due to the high false negative rate (FNR). In this study, we evaluated the usefulness of SLNB to confirm neck node metastasis in papillary thyroid microcarcinoma.
METHODS
We retrospectively reviewed medical records of 114 clinically T1N0 PTC patients who underwent thyroid surgery using blue dye in Pusan National University Yangsan Hospital from January 2012 to December 2013. T2 patients were excluded because they routinely underwent prophylactic central neck dissection. The study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (No. 05-2019-203) and performed in accordance with the principles of the Declaration of Helsinki. Informed consent was waived.
Operation procedure
All operations were performed by one experienced surgeon, who had annually performed more than 200 thyroid surgeries and had experience with thyroid SLN procedures.
Firstly, a transverse low-collar skin incision was performed, followed by the separation of skin flap and a longitudinal incision in the linea alba cervicalis. The thyroid capsule was then carefully opened to completely expose the thyroid gland without injuring the capsule, exposing both the thyroid gland and the ipsilateral jugular vein. SLNB was performed according to the method described by Hao et al. [8]. Subsequently, 0.5-mL Indigo carmine was peritumorally injected very slowly under low pressure by using a 1-mL syringe with a 27-gauge needle. Precaution was taken to avoid accidentally staining the surrounding structures. Lymph nodes that were stained blue within 3–4 minutes were defined as SLNs. Frozen section examination was then performed (Figs. 1, 2). If the frozen section was positive for SLNB, total thyroidectomy was performed.
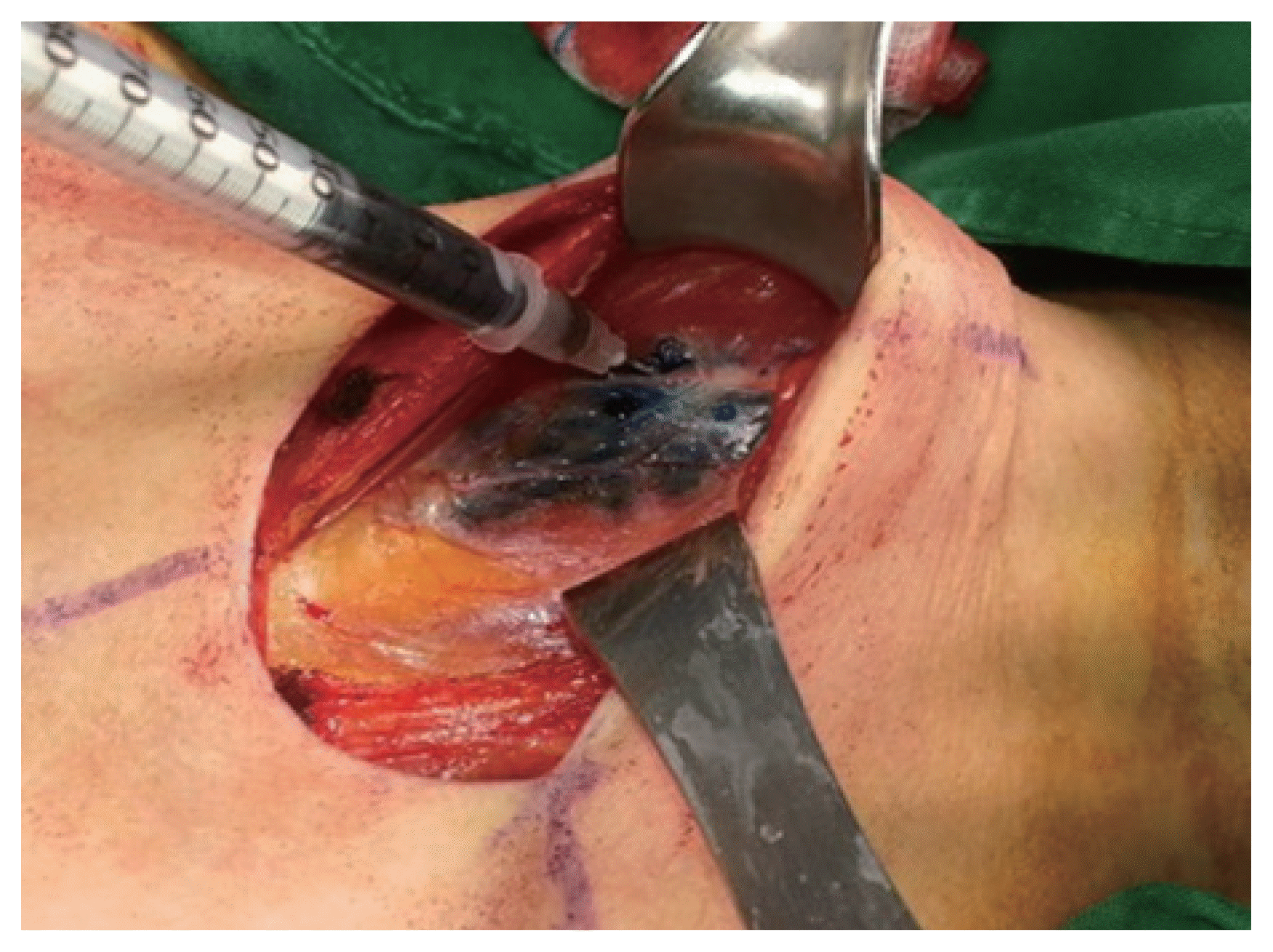
Injection of indigo carmine. Injection was done with 1-mL syringe for 5 seconds into the peri-tumoral space of thyroid.
Thereafter, the prophylactic ipsilateral central neck dissection followed by total thyroidectomy or unilateral thyroid lobo-isthmectomy was performed. The remaining unstained lymph nodes in the central compartment (non-SLNs) were sent for routine histopathology.
Statistical analysis
Statistical analyses were performed using software SPSS version 21.0 (IBM Corp., Armonk, NY, USA). The t-test and chi-square test were used. A P-value of less than 0.05 was considered statistically significant.
RESULTS
The mean age was 47 years and majority of the patients were female (98/114). The mean tumor size was 0.56 cm and the mean number of SLNs was 3.45. Total of 42 patients underwent total thyroidectomy and 72 patients underwent thyroid lobo-isthmectomy (Table 1).
SLNs were identified in 112 patients with 41 positive SLNs and 71 negative SLNs detected on intraoperative frozen sections. In the SLNs-negative group, the average age was 47 years, the average tumor size was 0.57 cm, the mean SLNs was 2.86 and the mean postoperative lymph nodes was 5.74. In the SLNs-positive group, the average age was 45 years, the average tumor size was 0.54 cm, the mean SLNs was 4.41 and the mean postoperative LNs was 11.46.
A univariate analysis was performed to determine whether these factors were independently correlated with SLN metastasis (SLNM). Male gender (P=0.004) and number of detected sentinel node (P=0.002) were independently predictive of SLNM (Table 2).
As mentioned earlier, SLNs were identified in 112 patients with 41 positive SLNs and 71 negative SLNs detected on intraoperative frozen sections. However, in the final pathologic reports, SLNM were found in 44 patients and non-SLNs were found in five patients. Three cases showed different results in the postoperative final report, from negative to positive sentinel node. Similarly, there were five cases of different results in non-SLNM. The sentinel node identification rate, false positive rate and FNR of SLNB were 98.2%, 0%, and 11.3%, respectively (Table 3). There was no recurrence in patients, except for eight patients who lost follow-up.
DISCUSSION
The role of routine central compartment neck dissection in the treatment of PTC remains unclear. There is no evidence in the literature to prove the general benefit of prophylactic neck dissection for PTC. Though some studies have suggested a decrease of regional recurrence and improvement of survival in high-risk patients [9,10], recent ATA guidelines do not recommend prophylactic central compartment neck dissection in T1 and T2 PTC patients with no clinical evidence of lymph node metastasis [3].
High-risk patients have a high likelihood of recurrence and reoperation rate. Reoperation is a psychological and economic burden for patients. Also, it is not only a complicated procedure but also has high incidence of complications [11,12].
Some surgeons perform selective central compartment neck dissection only when there is evidence of lymph node involvement. Others insisted that routine central neck dissection can reduce the local recurrence in high-risk patients [13].
SLNB is a standard staging procedure for the treatment of breast cancer and melanoma patients [13,14]. It is the basis of lymphatic dissection in the presence of metastasis and to avoid unnecessary surgery in the absence of metastasis. As a result, it protects the patient from the risks and costs of unnecessary surgery [6,7]. SLNB of PTCs has the potential to identify regional LN status without performing a prophylactic neck dissection and there are many studies on SLNB in PTC.
At present, SLNB is mainly traced by dye, radio-guided technique, or a combination of both techniques. Dye techniques are mainly employed, which are which are cheap and effective relatively. Radio-guided technique had an approximately 13% higher SLN detection rate compared to the dye technique [14]. Unfortunately, it is complicated, time consuming, expensive and caused radiological contamination. Furthermore, there is no obvious difference in sensitivity and specificity compared to the dye technique [15].
In this study, the SLNB was performed using the indigo carmine and the identification rate of sentinel node was 98%. However, there are several disadvantages of dye technique. First, lymphatic system can be damaged during SLNs tracing. Second, it is difficult to locate SLN within the lateral neck compartment. Finally, identifying a blue node is not always easy [16]. For these reasons, several studies have reported that the combination of dye and radiotracer has higher SLN identification and accuracy rate than the dye technique [17,18].
In recent years, with the development of nanotechnology, carbon nanoparticles have been widely used as lymph node tracer in malignant tumors. One study reported that carbon nanoparticle tracing showed better accuracy, sensitivity, specificity, and FNR than using dye [8].
In this study, the false positive rate of SLN and FNR of SLN were 0% and 11.3%, respectively. Among the 71 patients who were not diagnosed with SLNM, metastases were found in eight patients. In three cases, the SLN result changed from negative to positive and in non-SLN samples, five cases exhibited further metastases.
Despite the superior identification rate, accuracy, specificity and sensitivity, SLNB in PTC remain open to debate because of the high FNR. Also, an intraoperative pathological evaluation of SLN to avoid LND in patients with negative SLN is not recommended due to high FNR of the frozen section [19].
Previous studies have proposed that similar to breast cancer and melanoma, a radio-guided SLNB with SLN frozen section to identify patients with SLNM, LND of the neck compartments should be performed while those with negative SLN, LND may be avoided [20]. But, Carcoforo et al. [21] showed a high FNR of SLNB that are performed alone and the risk of remnant lymph node metastasis. Therefore, they have suggested a new concept of radio-guided surgery for lymph node staging of PTC patients by using SLNB as a guide to perform a selective LND of the SLN compartment regardless of the SLN status. This might be helpful for the final staging of disease and the reduction of persistent disease.
This study has three limitations. First, the study focused on the SLNs in the central compartment, in which the lymph node of lateral compartment was neglected. Second, this is a retrospective study only involving the papillary thyroid microcarcinoma. Third, this is a small single-center study conducted by an operator. Despite these limitations, we believe that this study will be helpful in future studies.
In conclusion, recent guidelines do not recommend prophylactic central compartment neck dissection in papillary thyroid microcarcinoma with no clinical evidence of lymph node metastasis [3]. However, we cannot exclude the possibility of persistent residual disease, which can increase the recurrence rate. SLNB is a surgical technique with high detection rate and low complication rate. Although large-scale prospective studies are needed, the application of SLNB is useful especially for male and high-risk patients. Considering the high FNR, it may be one of the treatment options for ipsilateral central neck dissection after SLNB.
ACKNOWLEDGMENTS
This study was supported by a 2021 research grant from Pusan National University Yangsan Hospital.
HYK and DIK designed and performed the research and wrote the paper; YJJ and SJL designed the research and supervised the report; CSJ and DWI designed the research and contributed to the analysis; JAY and JBC provided clinical advice; all authors read and approved the final manuscript.
Notes
No potential conflict of interest relevant to this article was reported.