Association between body composition measured by bioelectrical impedance analysis and platelet-to-lymphocyte ratio in colorectal cancer
Article information
Abstract
Purpose
This study investigated the relationship between body composition and platelet-to-lymphocyte ratio (PLR) in patients with colorectal cancer (CRC).
Methods
This retrospective study included 110 patients who underwent anthropometric measurement by bioelectrical impedance analysis before surgical treatment for CRC between May 2015 and June 2018.
Results
According to PLR, 45 patients (40.9%) had low PLR (PLR<150), and 65 patients (59.1%) had high PLR (PLR≥150). Serum hemoglobin (P<0.001) and albumin levels (P=0.021) were significantly lower in high PLR group. Tumor mass diameter was significantly larger in high PLR group (P=0.048) and the proportion of poorly differentiated or mucinous tumors was significantly higher in high PLR group (P=0.037). All indices related to fat (body fat mass, percent body fat, body fat mass of trunk, visceral fat area, fat mass index, measured fat thickness of abdomen) and two indices related to muscle (arm muscle circumference, measured muscle circumference of abdomen) were significantly lower in high PLR group (P<0.05). According to subgroup analysis based on the sex, all indices significantly differed between PLR groups; however, in females no index was significantly different between PLR groups.
Conclusion
Body composition indices including fat and muscle indices measured by InBody 770 were related to PLR in CRC, especially in male patients. These results suggest that low muscle and fat indices may be related to poor prognosis of CRC.
INTRODUCTION
Colorectal cancer (CRC) is third most frequently diagnosed malignancy in both sex groups worldwide. CRC incidence rates per 100,000 cases are 46.5 and 35.2 for males and females, respectively. For both sex groups, CRC is also the third leading cause of cancer-related deaths globally. Age-standardized mortality rates per 100,000 CRC-related deaths are 17.3 and 12.2 in males and females, respectively [1].
Inflammation and dysregulation of the coagulation system have been shown to play a role in tumor progression [2–5]. Platelet-to-lymphocyte ratio (PLR), which takes into account both the inflammatory and coagulation status, has been associated with the progression of CRC [6,7]. Recently, some studies have demonstrated that PLR might be useful for follow-up conversion of colonic and rectal neoplastic polyps to invasive tumors [8]. An elevated PLR was associated with poor prognosis of CRC, and thus PLR could serve as prognostic predictors in CRC patients [9].
Body composition analysis describes the percentages of fat, protein, minerals in human bodies. Bioelectrical impedance analysis (BIA) is a method used to determine body composition; it estimates impedance to alternating electrical current as it passes through the body of the user and is affected by the volume of water present in different tissue types. It is a noninvasive method and has very few side-effects.
Several studies have established a relationship between body composition and CRC. Fat composition of CRC patients is also known to affect cancer risk, prognosis, recurrence, and treatment toxicity [10]. Body muscle composition is also known to affect postoperative clinical outcomes, prognosis, and chemotoxicity of CRC [11–13]. Therefore, in our study, we aimed to investigate the relationship between body composition and PLR in patients with CRC.
METHODS
Patients
Using the medical records collected between May 2015 and June 2018, we retrospectively reviewed 605 consecutive cases of primary CRC where patients received surgical treatment. A total of 110 patients who underwent anthropometric measurement by InBody 770 (Biospace, Seoul, Korea) were included in our study, and the following exclusion criteria were considered for the selection of participating patients: (1) synchronous or previous malignancies; (2) malignancies other than adenocarcinoma; (3) refusal to undergo the BIA test; and (4) missing PLR score data. Finally, the selected population of 110 patients included 77 men (70.0%) and 33 women (30.0%). Using a prospectively collected database, information regarding patients’ demographics and hematological parameters (from blood samples) was obtained; this included data regarding age, sex, history of smoking and drinking, American Society of Anesthesiologists grade, height, weight, sidedness and location of the tumor, and preoperative carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels. This study protocol was approved by the Institutional Review Board of the Dongsan Medical Center (IRB No. 2018-12-024), and informed consent was obtained from all patients.
Preoperative evaluation and surgical treatment
All of the patients underwent a colonoscopy, biopsy, and staging scan (including computed tomography of the chest, abdomen, and pelvis, and magnetic resonance imaging of the pelvis). In addition, positron emission tomography scans and endorectal ultrasounds were carried out in few patients. Following the original description [14], we applied the general principles of complete mesocolic or mesorectal excision and central vascular ligation for CRC. The primary tumor was resected by sharp dissection of the visceral plane from the parietal fascia layer along with the entire regional mesocolon in an intact package. For right-sided colon cancer, radical lymphadenectomy was performed along the primary feeding vessels following a vertical line to expose the superior mesenteric vein. For left-sided colon or rectal cancer, high or selective ligation of the inferior mesenteric artery along with the lymph node dissection was performed based on the tumor location. Tumor stages were classified according to the guidelines of the American Joint Committee on Cancer staging system, 6th edition.
Assessment of hematologic parameters and inflammation-based prognostic scores
During the outpatient visit, patients’ blood samples were collected just before their surgery to investigate the hematologic parameters, including hemoglobin, white blood cell (WBC), platelet, and albumin. A complete blood cell count was performed on these blood samples to calculate the PLR. The PLR was calculated as the absolute count of platelets divided by the absolute count of lymphocytes. Patients were divided into the low and high PLR groups using a cutoff value of 150 [9]. In addition, other inflammation-based prognostic scores were calculated (prognostic nutritional index [PNI]: 10×serum albumin concentration (g/dL)+0.005×absolute lymphocyte count; neutrophil lymphocyte ratio [NLR]: absolute neutrophil count/absolute lymphocyte count).
Bioelectrical impedance analysis
Using InBody 770 (Biospace), a BIA test was performed at the patient’s first visit to estimate their body composition. Further, we studied the various parameters of BIA and generated the following categories of measured variables: body composition or metabolic index, fat index, and muscle index.
Statistical analysis
All statistical analyses were performed using SPSS statistical software version 25.0 (IBM Corp., Armonk, NY, USA). The results were presented as means with standard deviations (mean±SD) for continuous outcomes, and as frequencies and percentages for categorical outcomes. Categorical variables were analyzed using the chi-square and Fisher exact tests. Continuous variables were analyzed with independent t-test and Mann-Whitney test. A P-value of <0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
This study included 110 CRC patients, and the baseline characteristics of these patients are summarized in Table 1. Their mean age was 68.3±9.6 years, and 77 of 110 patients (70.0%) were male. There were 74 patients (67.3%) with left-sided CRC and 38 (34.5%) with rectal cancer. Patients’ mean CEA and CA19-9 levels were 3.80±4.93 and 13.45±13.27, respectively. According to PLR, 45 patients (40.9%) had a low PLR (PLR<150), while 65 patients (59.1%) had a high PLR (PLR≥150). Patient’s age, sex, smoking status, drinking status, American Society of Anesthesiologists score, tumor’s location, CEA level, and CA19-9 level did not differ significantly between both PLR groups. In contrast, relative to the low PLR group, the high PLR patient group exhibited significantly greater height (P=0.043) and lower weight (P=0.014).
Hematological parameters and inflammation-based prognostic scores
Hematological parameters according to the PLR are presented in Table 2. Relative to the low PLR group, serum hemoglobin (P<0.001) and albumin (P=0.021) levels were significantly lower in the high PLR group patients. WBC level was not significantly different between the two groups; however, there were significant differences in the platelet level (P<0.001), and the proportion of neutrophil (P<0.001) and lymphocyte (P<0.001) in WBC. Other inflammation-based prognostic scores were also calculated for the PLR groups. The PNI score was significantly lower in the high PLR group (P<0.001), while the NLR score was significantly higher in the high PLR group (P<0.001).
Pathological characteristics
Pathological findings of patients according to PLR are provided in Table 3. The largest dimension of the tumor mass was overall greater in the high PLR group (P=0.048). Well or moderately differentiated tumors were the predominant type in both PLR groups; however, the proportion of poorly differentiated or mucinous tumors significantly increased in the high PLR group (P=0.037). The high PLR group appeared to have more advanced T stages, although these differences were not statistically significant (P=0.097). In present study, two patient groups did not exhibit statistically significant differences in N stage, lymphovascular invasion, tumor budding, perineural invasion, and extranodal tumor deposit.
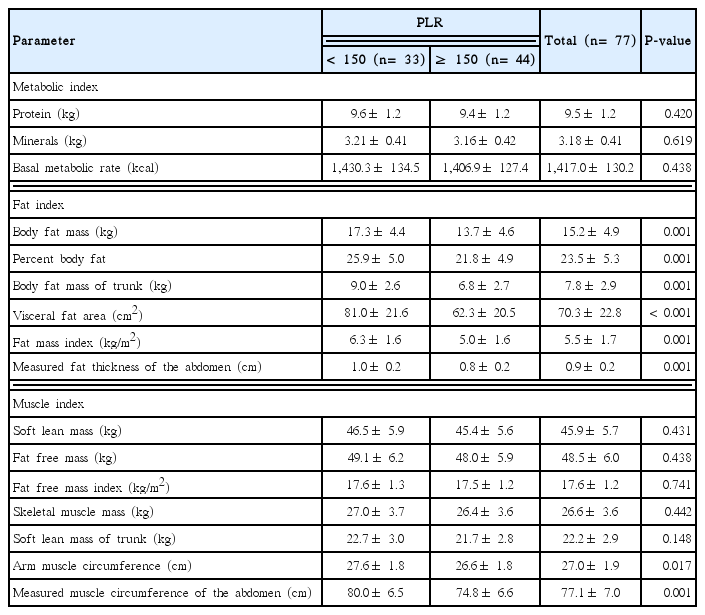
Body composition indices according to PLR
The results of body composition analyses of the patients are summarized in Table 4. We classified our results in three categories: body composition or metabolic, fat, and muscle indices. Body composition or metabolism indices did not significantly differ between both PLR groups. However, all indices related to fat (body fat mass, percent body fat, body fat mass of trunk, visceral fat area, fat mass index, and measured fat thickness of the abdomen) and two indices related to muscle (arm muscle circumference and measured muscle circumference of the abdomen) were significantly lower in the high PLR group (P<0.05).
The results of body composition analysis according to sex groups are described in Tables 5 and 6. In male participants, all indices which were significantly different in Table 4 remained statistically significant and same tendency between PLR groups. In female patients, none of the measured indices were significantly different between the two PLR groups.
DISCUSSION
The present study shows that among CRC patients, all fat and two muscle indices (measured by InBody 770) were significantly low in the high PLR group, especially in males. In the high PLR group, the NLR score was significantly high while the PNI score was significantly low. These results suggest that body composition indices are closely related to PLR, which is known to be associated with prognosis of CRC.
Previous studies have reported that body composition may play a critical role in carcinogenesis and prognosis of CRC. Karahalios et al. [10] demonstrated that a 5-kg weight gain was associated with a 3% increased risk of CRC, and this association was slightly stronger for men than women. Bardou et al. [15] identified visceral adipose tissue as a potential risk factor for CRC (risk ratio, 1.9; 95% confidence interval, 1.1–3.3), suggesting its strong correlation with colorectal adenoma prevalence independent of body mass index (BMI). Additionally, visceral adipose tissue may serve as a more accurate marker than the waist circumference for assessing increased CRC risk. Comparing participants with sarcopenia to those without sarcopenia, the odds ratio (95% confidence interval) was found to be 1.99 (1.63–2.43) for advanced CRC patients [16]. Lieffers et al. [13] reported that sarcopenia is associated with postoperative infections and inpatient rehabilitation care, consequently resulting in a prolonged hospital stay. For CRC, sarcopenic obesity is a predictor of severe postoperative complications after open colon resection and further relates to chemotherapy toxicity [11].
Previously, studies have reported that inflammatory and nutritional indices including PNI, NLR, and PLR are related to prognosis of CRC, and these indices are further related to each other [17]. Preoperative PLR was associated with poor overall survival, disease-free survival, cancer-specific survival, and recurrence-free survival [17]. Additionally, preoperative PNI was a useful predictor of postoperative complications and survival outcomes in patients with CRC [18]. Li et al. [19] reported that elevated pretreatment NLR predicted poor overall survival and differentiation of the tumor. In the present study, PNI was significantly low, and NLR was significantly high in the high PLR group. These results are consistent with the findings of the previous studies.
In the present study, high PLR group patients had large mass size and poorly differentiated tumors; however, there was no significant relationship between other pathologic findings and PLR in our CRC patients. Previous studies have shown that elevated PLR is a poor predictor of clinicopathologic features in CRC patients [20]. Ozawa et al. [6] observed large tumor mass size and a high proportion of poorly differentiated tumors in the high PLR group with stage II CRC. Further, Park et al. [12] demonstrated that T stage, vascular invasion, and perineural invasion were significantly high in the high PLR patients.
Several studies have observed that arm circumference is related to inflammatory responses and nutritional status of patients with cancer [18]. However, to our knowledge, there exists only one study that analyzed the relationship between PLR and body composition measured by BIA. Liaw et al. [21] reported that PLR levels exhibit an independent negative association with the skeletal muscle index measured by BIA. Since then, there have been no studies conducted to investigate the relationship between PLR and body compositions measured by InBody 770, a new version of InBody. InBody 770 has unique body composition indices including arm muscle circumference and measured muscle circumference of the abdomen that might be considered superior to previously used anthropometric indices such as mid-upper arm circumference. In our study, low arm muscle circumference and measured muscle circumference of the abdomen were related to a high PLR, especially in the male group; however, further study is needed to validate these results.
Interestingly, in this study, low fat indices were exclusively related to high PLR in the male group. There have been no studies conducted on the relationship between fat compositions and PLR. However, previous studies have reported platelet and lymphocyte counts to be associated with fat compositions in the body. Bahadir et al. [22] reported that there is a significant increase in lymphocyte count with increasing BMI. Further, Samocha-Bonet et al. [23] found that platelet count was positively correlated with BMI only in females. Females have high body fat mass and excessive adipose tissue that can together induce systemic and chronic inflammation through the release of inflammatory cytokines including interleukin 6 [24]. These inflammatory cytokines play a crucial role in increasing the platelet count. We believe that our results regarding fat indices showing sex differences may be suggestive of a positive correlation between platelet count and BMI in females. However, this observation should be interpreted with caution due to the small sample size of female participants included in this study.
We acknowledge that this study had some limitations worth noting. Firstly, this study was retrospectively performed at a single center. Secondly, it was difficult to avoid a selection bias when collecting information on patients with CRC. Thirdly, our study included a small number of patients and lacked their survival data due to short follow-up period. Additionally, we failed to obtain patient data regarding metabolic diseases such as diabetes mellitus, dyslipidemia, hypertension, and liver diseases.
In summary, this study demonstrated that InBody 770 body composition indices including fat and muscle indices were related to PLR in CRC patients, especially in males. These results suggest that preoperative low muscle and fat indices may be related to poor prognosis of CRC.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (No. 2017R1C1B5076880).
Notes
This study was presented at the Annual Congress of KSS 2018, on November 1–3, 2018, in Seoul, Korea.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.