Comparison of early and late surgery following colonic stenting for obstructive colorectal cancer
Article information
Abstract
Purpose
Colonic stenting as a bridge to surgery is an alternative to emergency surgery in patients with acute malignant colonic obstruction. This study aimed to compare the outcomes of early and late surgery after colonic stenting for obstructive colorectal cancer.
Methods
From March 2004 to August 2014, the medical records of obstructive colorectal cancer patients who underwent surgery after colonic stent insertion were retrospectively reviewed. The patients were divided into early surgery (≤7 days after stenting) and late surgery (>7 days after stenting) groups.
Results
Eighty-four patients underwent colonic stenting for obstructive colorectal cancer. Forty-six patients were ultimately enrolled: 18 in the early and 28 in the late surgery groups. The mean ages were 63.7 and 71.8 years, respectively (P=0.01). Blood loss was lower in the early surgery group (median [interquartile range], 50 [50–50] mL vs. 50 [50–100] mL; P=0.020). The time to first flatus was longer in the early surgery group (3.0 [3.0–5.0] days vs. 2.0 [2.0–3.0] days; P=0.010). The time to first soft food intake was similar. Postoperative complications did not differ (16.7% vs. 14.3%, respectively; P=0.525) and no patients died.
Conclusion
Surgical outcomes were similar between early and late surgery. However, the former featured less blood loss, indicating less surgical difficulty. These results show that early surgery can be performed safely in obstructive colorectal cancer patients after colonic stenting if the patient’s clinical condition is amenable to early surgery.
INTRODUCTION
An estimated 10%–30% of colorectal cancer patients suffer from colon obstruction, which can lead to pathological distention, bacterial infection, electrolyte imbalance, necrosis, and colon perforation [1]. Emergency surgery, an effective treatment option for acute malignant colonic obstruction, consists of tumor resection and stoma creation, followed by later stoma reversal. Emergency surgery, however, is associated with a high morbidity rate of up to 40% [2]. Additionally, stoma creation decreases patient quality of life.
Colonic stenting as a bridge to surgery is an alternative to emergency surgery in patients with acute malignant colonic obstruction. Colonic stenting alleviates acute obstruction, helps surgeons avoid emergency surgery, and enables preoperative bowel preparation for elective surgery. Therefore, it provides an opportunity for patients with obstructive colorectal cancer to undergo single-stage surgery with primary bowel anastomosis and avoid stoma-related complications and inconvenience [3]. Moreover, colonic stenting has shown no adverse effect on perioperative mortality, long-term survival, or progression of colorectal cancer [4].
Despite the widespread use of colonic stenting, the optimal interval between colonic stenting and surgery after achieving bowel decompression is controversial. Few studies have investigated the interval [5]. This study aimed to compare the outcomes of early versus late surgery after colonic stenting for obstructive colorectal cancer.
METHODS
Study population and design
From March 2004 to August 2014, the medical records of obstructive colorectal cancer patients who underwent surgery after colonic stent insertion at Keimyung University Dongsan Medical Center were retrospectively reviewed. The exclusion criteria were as follows: age < 20 years, severe comorbidity as judged by an American Society of Anesthesiologists (ASA) score of 4, surgery with palliative intent, history of preoperative chemotherapy or radiotherapy, iatrogenic colonic perforation during colonic stenting, and failed colonic decompression after colonic stenting.
In the European guidelines, a 5- to 10-day interval between colonic stenting and elective surgery was suggested [5]. The suggested time interval was thought to be too broad for clinical implementation and required to become more explicit. Hence, we decided to compare the time intervals of within 7 days versus after 7 days. The patients were divided into two groups: surgery = 7 days after stenting (early surgery group) and surgery > 7 days (late surgery group).
The two groups were compared in terms of patient demographics, clinical data, and perioperative outcomes. Postoperative morbidity and mortality were defined as an adverse event and death occurring within 30 days after colorectal surgery, respectively. Data on the following postoperative complications were collected and analyzed: surgical and non-surgical infections, urinary complications, and anastomotic leakage. Anastomosis leakage was defined as the symptoms and signs of peritonitis caused by anastomotic dehiscence, which was confirmed by abdominal computed tomography scan imaging.
Colonic stenting and bowel preparation
The colonic stents were inserted under fluoroscopy guidance by endoscopists with relevant experience and expertise. Successful colonic stenting was defined as complete stent coverage of colonic stenosis, passage of stool, and relief of the bowel obstruction as assessed by the resolution of clinical symptoms and radiological signs.
The patients who were judged as having successful colonic stenting received mechanical bowel preparation using polyethylene glycol starting 2 days before surgery.
Statistical analyses
Statistical analyses were performed with PASW Statistics ver. 18 software (SPSS Inc., Chicago, IL, USA). The data are displayed as frequencies and percentages for categorical variables and were analyzed with the Pearson’s chi-square test or Fisher exact test. The Kolmogorov-Smirnov test was used to test the distribution of continuous variables. Normally distributed variables were subjected to examination with Student’s t-test and the results are expressed as mean± standard deviation. Non-normally distributed continuous variables were examined with the Mann-Whitney U test and the results are presented as median (interquartile range). The Ka-plan-Meier method was used to analyze survival. Two-tailed values of P< 0.05 were considered statistically significant.
RESULTS
Demographics and clinical findings
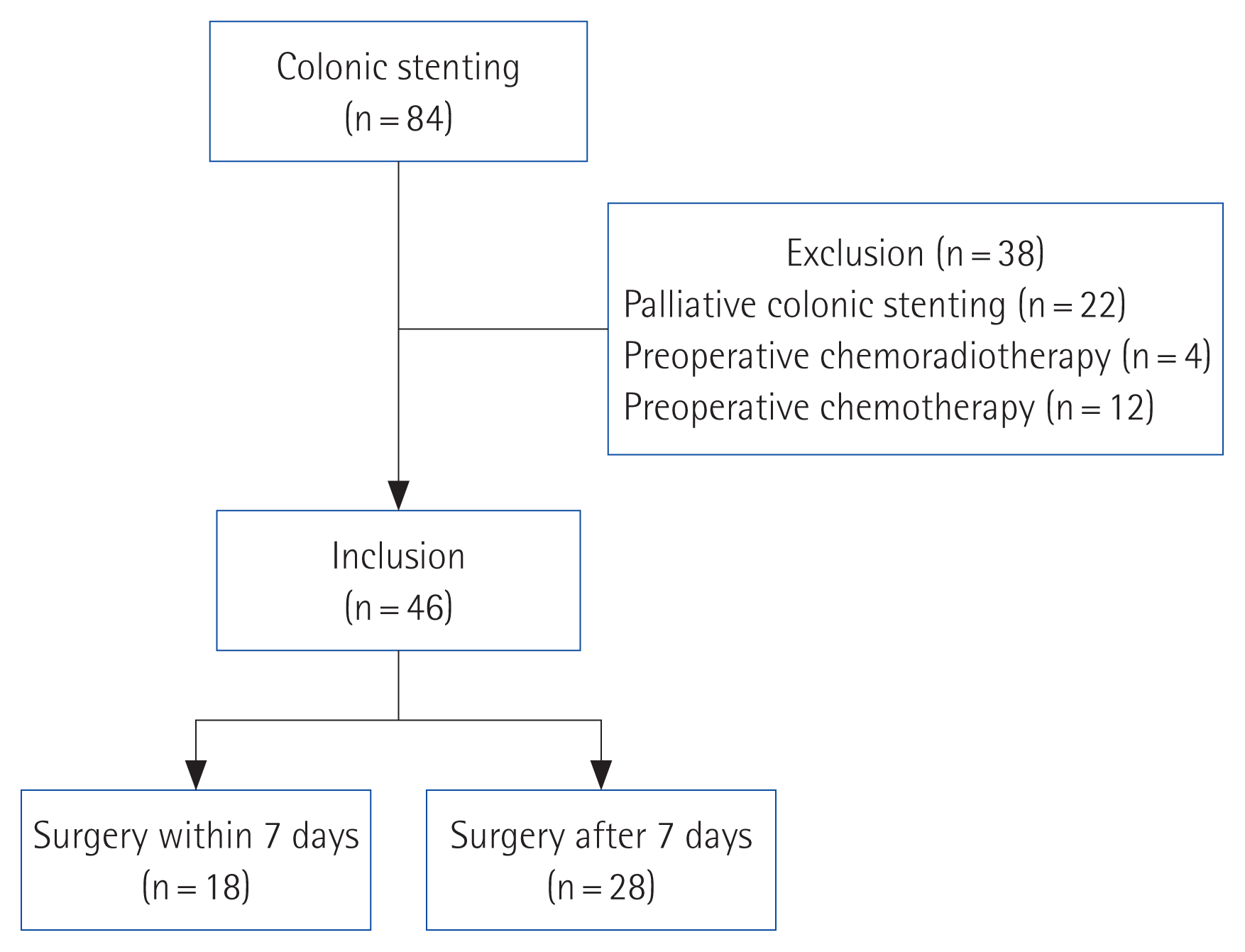
A total of 84 patients underwent colonic stenting for obstructive colorectal cancer. Patients who received colonic stenting for palliative purposes (n= 22) or received preoperative chemoradiotherapy (n= 4) or chemotherapy (n= 12) after stent insertion were excluded. Forty-six patients were enrolled in this study. Of them, 18 were assigned to the early surgery group and 28 were assigned to the late surgery group (Fig. 1).
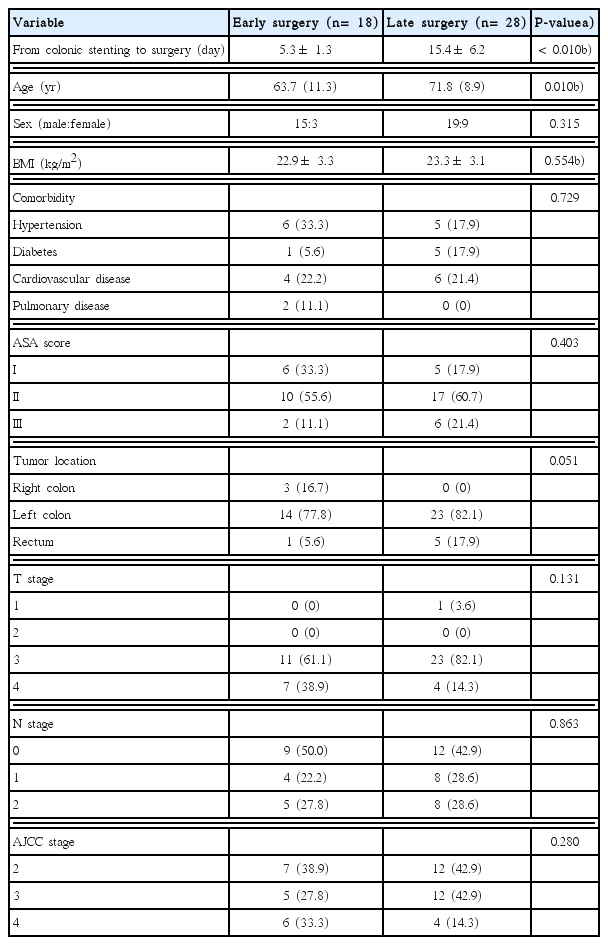
The mean interval from colonic stenting to surgery between the two groups was significantly different (5.3± 1.3 days vs. 15.4± 6.2 days, respectively; P< 0.010). The interval ranges of two groups were 3 to 7 days and 8 to 33 days, respectively. The mean age of the early surgery group was younger than that of the late surgery group (63.7 years vs. 71.8 years; P= 0.010). There was no significant difference in sex ratio, body mass index, comorbidities, ASA score, tumor location, T stage, N stage, or American Joint Committee on Cancer stage (Table 1).
A total of 10 patients with stage IV colorectal cancer underwent surgery for primary colorectal cancer (six in the early group and four in the late group). Combined resection for metastatic lesions was performed simultaneously in three patients (two in the early group and one in the late group). One patient in the late surgery group underwent radiofrequency ablation for liver metastasis (Table 2).
Perioperative results and complications
Operations, surgical approach, stoma rate, and operation time did not differ significantly between the two groups (Table 3). No conversions from laparoscopic to open surgery or from robotic to laparoscopic surgery occurred.
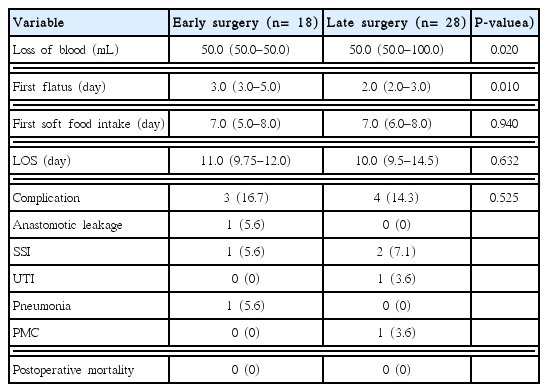
The postoperative course and complications are shown in Table 4. The degree of blood loss was lower in the early surgery group than in the late surgery group (median [interquartile range], 50 [50–50] mL vs. 50 [50–100] mL; P= 0.020). The time to first flatus was longer in the early surgery group (3.0 [3.0–5.0] days vs. 2.0 [2.0–3.0] days; P= 0.010). However, the time to first soft food intake (7.0 [5.0–8.0] days vs. 7.0 [6.0–8.0] days; P= 0.940) and length of stay (11.0 [9.75–12.0] days vs. 10.0 [9.5–14.5] days; P= 0.632) were similar. No significant difference was seen in postoperative complications (16.7% vs. 14.3%, respectively; P= 0.525). Anastomosis leakage occurred in one patient in the early surgery group who underwent anterior resection and a right lobectomy for sigmoid colon cancer with liver metastasis 3 days after colonic stenting (patient 4 in Table 2). On postoperative day 7, anastomosis leakage was diagnosed and managed with resection of the anastomosis and end colostomy. A total of three patients experienced a surgical site infection (SSI). One in each group had a superficial incisional SSI, while one patient in the late surgery group had organ/space SSI and underwent percutaneous drainage. No case of mortality occurred in either group.
Cancer recurrence, disease-free survival, and overall survival
During a median follow-up period of 30 months (range, 7–97 months), the disease recurrence rate was not different significantly between the two groups (3/18, 16.7% vs. 9/28, 32.1%; P= 0.315). Systemic recurrence occurred in three and seven patients in these groups, respectively. Two patients in late surgery group experienced local recurrence (Table 5).
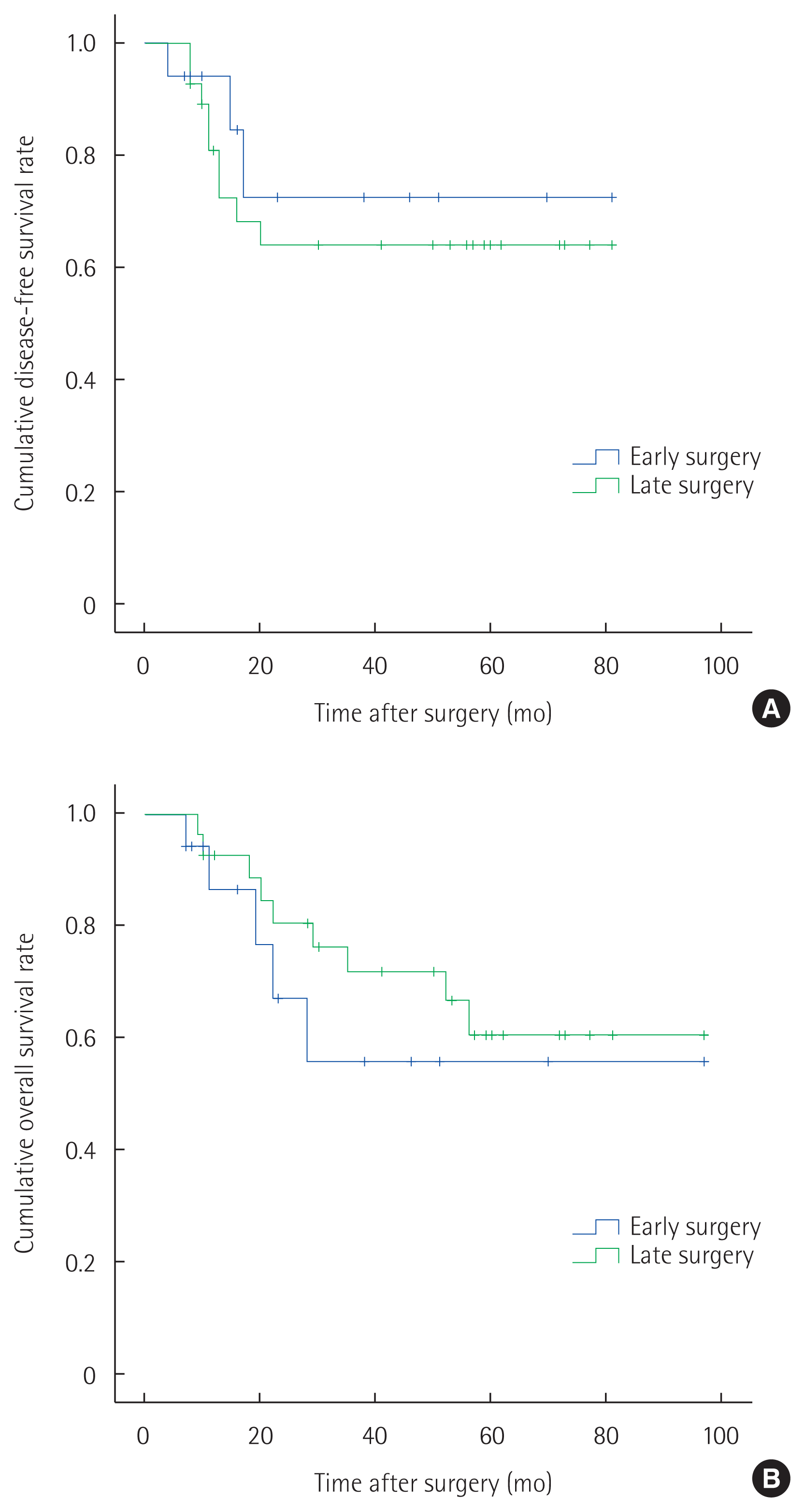
The 5-year disease-free survival rate was similar in the early and late surgery groups (72.6% and 64.0%, respectively; P= 0.558) (Fig. 2A). The 5-year overall survival rate was lower in the late than early surgery group, but the difference was not significant (56.1% vs. 60.8%, respectively; P= 0.437) (Fig. 2B).
DISCUSSION
The optimal timing of surgery after colonic stenting has not been established. In the European guidelines, the suggested time interval between colonic stenting and surgery is 5–10 days when colonic stenting is used as a bridge to elective surgery in patients with potentially curable left-sided colon cancer [5]. The time interval in the guideline was determined based on a few studies with different qualities. For this reason, the recommendation on the time interval is weak and the level of evidence is low. In a meta-analysis comparing colonic stenting as bridge to surgery and emergency surgery for left-sided colorectal cancer obstruction, surgery was generally performed after a median 10 days after colonic stenting [6]. This study, however, did not aim to determine the optimal time interval between colonic stenting and surgery. Moreover, the median 10 days resulted from statistical analysis of the data from the included studies. A retrospective study concluded that a duration of 7–9 days after colonic stenting in patients with obstructive left-sided colon cancer is sufficient to subsequently perform a safe surgical procedure. This study, however, has limitations for drawing the conclusion. It was a single arm study that did not compare the patient groups according to the time interval after colonic stenting whose conclusion was supported by limited and logically flawed descriptions [7]. Thus, well-designed, highly reliable studies are required to conclude the optimal timing of surgery after colonic stenting.
In this study, the patients in the early surgery group were younger than those in the late surgery group. It is a common belief that the general health and performance status of young patients are better than those of older ones. Older patients usually require more preoperative examinations. Nonetheless, age does not usually influence the timing of surgery after colonic stenting. In our previously published study comparing the postoperative outcomes of obstructive colorectal cancer patients aged < 70 years and those aged = 70 years, the time interval between colonic stenting and surgery did not differ significantly between the two groups and the postoperative complications were similar [8]. According to the results of the previous study, the difference in age between the early and late surgery groups is not thought to affect the comparison of the postoperative outcomes. In addition, comorbidities, ASA score, and other variables influencing on the preoperative checkup and postoperative complications, did not differ between the two groups in the present study.
The operation time and blood loss are variables representative of surgical difficulty [9]. In this study, the operation time did not differ significantly between the two groups. Estimated blood loss was lower in the early surgery group, which indicates a lower degree of surgical difficulty. These results show that early surgery can be performed without increased surgical difficulty.
The postoperative course of the two groups was similar in this study. Despite the early surgery group having a longer time to first flatus, no significant differences were found in the time to first soft food intake or, eventually, length of stay.
The postoperative complication rate is similar between two groups (16.7% vs. 14.3%, respectively; P= 0.525). The complication rate (7/46, 15.2%) was consistent with those of other studies of 8.5%–25% [8,10,11].
Anastomosis leakage of the most concerning complications of surgery following colonic stenting. Incomplete resolution of bowel wall edema is thought to contribute to the occurrence of anastomosis leakage. The time interval after colonic stenting to achieve an appropriate bowel thickness for safe anastomosis, although not well known, is influenced by many factors such as stent expansion, obstructive bowel length, obstruction duration, and nutritional status. In the present study, one patient who underwent anterior resection 3 days after colonic stenting with a simultaneous right lobectomy for liver metastasis developed anastomosis leakage on postoperative day 7. Thus, 3 days might be an insufficient time for the normal physiology of the bowel to resume. Additionally, a wide range of surgical interventions would be another risk factor for anastomosis leakage. Except for that one patient, no other patients in the early surgery group developed anastomosis leakage regardless of stoma status. In the present study, achieving a suitable bowel status for a safe anastomosis was possible within 7 days. The rates of anastomosis leakage in all patients and those without a stoma were 2.2% (1/46) and 2.7% (1/37), respectively. These rates are consistent with those of other studies (range, 0%–10.6%) [6–8].
In this study, the surgical timing did not affect oncologic outcomes. No significant differences were noted in 5-year disease-free and overall survival rates. In the literature, few studies have analyzed the effect of surgical timing after colonic stenting on oncologic outcomes. Oncologic outcomes between elective surgery following colonic stenting and emergency surgery are controversial. Some studies reported comparable oncologic outcomes of elective surgery after colonic stenting to emergency surgery [12–14]. In contrast to our findings, worse oncologic outcomes of colonic stenting were published [15,16]. These outcomes are mainly associated with tumor cell dissemination caused by tumor perforation related to colonic stenting and stent placement [16–18]. A longer interval between colonic stenting and surgery may increase the risk of stent-related complications including tumor perforation [5]. In the present study, no tumor perforation or other stent-related complications occurred in enrolled patients regardless of the surgical timing, which might be a reason for the lack of oncologic differences between the two groups.
There are limitations of the present study. First, it was not a prospective controlled study, so it carries the risk of selection bias. Second, its relatively small number of enrolled patients may reduce the power of its results.
In conclusion, outcomes were similar between early and late surgery patients. However, early surgery patients had less blood loss, which indicates a lower degree of surgical difficulty. These results show that early surgery can be performed safely to treat obstructive colorectal cancer patients after colonic stenting if the patient’s clinical condition is amenable to early surgery.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.