췌장수술 후의 백혈구 수치 변화: 췌장루의 조기 징후인가?
Postoperative white blood cell counts change after pancreatoduodenectomy: Early sign for pancreatic fistula
Article information
Trans Abstract
Purpose:
Pancreatic surgery is challenging and associated with high morbidity. Therefore, it is important to detect it early before it becomes clinically apparent. The white blood cell (WBC) count useful as a predictive marker of postoperative pancreatic fistula. The aim of this study was to evaluate the diagnostic accuracy of WBC in predicting pancreatic fistula.
Methods:
Between September 2003 and December 2013, 405 patients underwent elective pancreaticoduodenectomy or pylorus preserving pancreaticoduodenectomy for periampullary malignancy. Among them, 372 patients with no preoperative leukocytosis were enrolled in this study. The serum WBC count was monitored daily until postoperative day 8. The clinic and pathological data of these patients were analyzed by reviewing medical records retrospectively.
Results:
Thirty patients (8%) developed pancreatic fistula grade B and C. The WBC count, measured every other day, was significantly higher every other day during the first 7 postoperative days in patients who developed pancreatic fistula grade B and C, compared with those patients who did not develop pancreatic fistula. The WBC count cutoff value of 13.07×109/L, 10.37×109/L on postoperative day 1,7 yielded a sensitivity of 57%, 70%, specificity of 53%, 67% for the detection of pancreatic fistula.
Conclusion:
Patients with postoperative fistula grades B and C showed WBC counts that did not decrease on subsequent measurements during the early postoperative period. The measurement of WBC counts after pancreaticoduodenectomy can play a clinically important role in the early detection of pancreatic fistula development even from postoperative one day.
INTRODUCTION
Pancreaticoduodenectomy (PD) or Pylorus preserving pancreaticoduodenectomy (PPPD) are technically challenging surgical procedures that require a certain high level of experience and techniques with regard to resection and reconstruction [1]. The rate of mortality of PD and PPPD is less than 5%. However the morbidity has been reported still high almost 40%–50% [2,3]. One of the most important life-threatening complications is a postoperative pancreatic fistula (POPF) [4,5]. As there are few specific and early sign of POPF, it is occasionally diagnosed late in the postoperative period after patients develop peritonitis or sepsis [6]. The delayed diagnosis may lead to severe fatal status. Therefore it is important to detect these complications as early as possible.
Several biochemical tests have been proposed to detect inflammatory activity postoperatively, including serum levels of C-reactive protein, procalcitonin, interleukins, and white blood cells (WBC) [7-11]. The WBC count has been widely used because of its simplicity, availability, and low cost. The WBC count is a non-specific inflammatory mediator with a variant half-life (6 hour–few days). It is a valuable marker for most forms of inflammation like pancreatitis, tissue damage, tissue ischemia or necrosis [4,6,12-14]. Several studies reported leukocytosis on postoperative day (POD) 4 or 5 is related with pancreatic anastomotic leakage after PD [13]. However, there are a few reports on the relationship of leukocytosis, neutrophil to lymphocyte ratio (NLR) and POPF in immediate postoperative days [15]. Although leukocytosis may be common postoperative finding, it would be very beneficial to establish the correlation of WBC count and NLR to the development of POPF. Therefore, present study was designed to evaluate the usefulness postoperative serial WBC and/or NLR count for the early prediction of POPF.
METHODS
Patients
The patients who underwent PD (or PPPD) for periampullary malignancy at the Seoul National University Bundang Hospital, from September 2003 and December 2013 were included in this retrospective study. Four hundred five patients were included and their preoperative clinical data (age, sex, body mass index, mellitus diabetes, preoperative drainage, preoperative jaundice, preoperative total bilirubin level, preoperative albumin level, preoperative WBC count), intraoperative finding (tumor location, type of operation, operation time, estimated blood loss, pancreatic duct size, pancreatic duct dilatation, consistency of pancreas, transfusion of red blood cell), pathologic finding (tumor location, T stage, N stage, resection margin), postoperative clinical data (WBC count, neutrophil count, lymphocyte count, drain amylase level, serum amylase level) and also postoperative course included complications (POPF A-B-C according to International Study Group on Pancreatic Fistula classification [6], intra-abdominal fluid collection, intra-abdominal bleeding, pseudoaneurysm, atelectasis, pleural effusion, pneumonia, wound problem, delayed gastric emptying and ileus) were collected from electromedical record. These complications are categorized by Clavien-Dindo classification [6].
Perioperative care
Two Jackson-Pratt (JP) drains were placed around pancreaticojejunostomy site. Routine postoperative follow up abdominal computed tomography (CT) scan was performed at POD 7. If the patients had peritoneal irritation sign with fever [16], immediate CT scan was performed. The amount of drainage was recorded daily till removal of JP drains. The serum amylase and drainage amylase level was checked at POD 3, 5, and 7. These tests were repeated every other day till removal of JP drain.
Exclusion criteria
The exclusion criterion was preoperative leukocytosis (WBC ≥10.00×109/L). After excluding 33 patients with preoperative leukocytosis, 372 people were enrolled and analyzed. Diagnosed periampullary cancers were pancreatic head cancer, distal common bile duct cancer, Ampulla of Vater cancer and duodenal cancer.
Complications
The postoperative complications were defined as follows: POPF, fluid collection, bleeding or pseudoaneurysm, infection (lung problems or wound problem), non-infectious complications (ileus, delayed gastric emptying) and others. The grading of POPF was defined by classification of International Study Group on Pancreatic Fistula. POPF was classified into grades: grade A-pancreatic fistula (PF) which is mild form, and grades B,C-PF were severe [6]. The subjects included were divided into: No leakage or no PF, Minor leakage or A-PF, and Major leakage composed of B and C-PF.
Statistical analysis
The resulting data was analyzed using PASW statistics 18 for Windows. Demographic data was expressed as mean. The statistical significance of the continuous variables was examined by a Student’s t-test or Mann–Whitney’s U test, χ2 test or Fisher’s exact test. Analysis of variance was used for categorical data analysis. The diagnostic accuracy of WBC count was assessed by receiver operating characteristics (ROCs) curve analysis. This method calculates the sensitivity and specificity of each observed test result with regard to a defied classification variable, identifying the cutoff value for WBC count with the highest sensitivity and specificity. A ROCs curve was obtained by plotting the sensitivity (fraction of true positives, y-axis) against 1-specificity (fraction of false negatives, x-axis). The area under the curve (AUC) was a direct measure of the diagnostic accuracy of the test. The AUC value 50% indicates the ability of a test to significantly discriminate between positive and negative cases with regard to the classification variable (e.g., presence or absence of POPF B-C). The P-value <0.05 (two-sided tests) was considered statistically significant. Simple linear or multiple linear regression analysis was also performed to calculate adjusted odds ratios and 95% confidence intervals (95% CI).
RESULT
Characteristics
The Table 1 shows the clinical characteristics of the study population. Two-hundred and seventeen (58.3%) patients were male and 155 (41.7%) were female, with an average age of 67±10.1 years. Among 372 patients, 161 (43.2%) had pancreatic head cancer, 108 (29%) had distal common bile duct cancer, 86 (23%) had Ampulla of Vater cancer and 17 (4.5%) had duodenal cancer. Three hundred ten (83.3%) patients underwent PPPD and 62 (16.7%) patients underwent PD. Ninety seven (26%) patients developed PF. The number and incidence of each grade: Minor leakage and Major leakage was 67 (18%) and 30 (8%). There were no statistically significant differences between No leakage, Minor leakage, and Major leakage.
Other complications
One hundred four patients (27%) had other complications such as fluid collection (21 patients), bleeding or pseudoaneurysm (18 patients), infection: lung or wound (34 patients), non-infection: ileus, delayed gastric emptying (31 patients). Among them, 48 patients had been treated with interventional treatment or more (Table 2). Three patients died within 30 days after surgery (overall mortality 0.8 %); one from a disseminated intravascular coagulation and the other two from multi-organ failure.
Pancreatic fistula
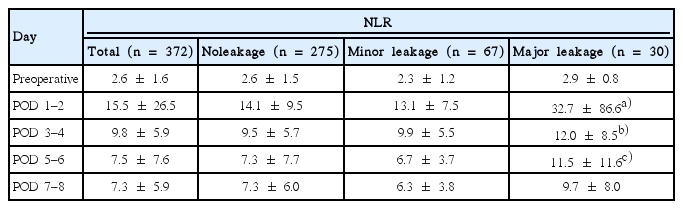
There was a no statistically significant difference between No leakage and Minor leakage on WBC levels and NLR. Major leakage had higher WBC values and NLR than No leakage. Statistically difference was observed on the average values of WBC from POD 1 to 8. In No leakage and Minor leakage, WBC peaked on POD 1, and gradually decreased thereafter. In Major leakage, median value of WBC also peaked on POD 1. WBC count decreased gradually slowly during the subsequent days and then increased again at POD 7-8 (Fig. 1). Major leakage had significantly higher WBC levels during POD 8 (Table 3). In addition, NLR was higher in Major leakage, the difference were statistically significant (Table 4). ROCs analysis was performed to define the predictive WBC value of Major leakage. On POD 1, a cutoff value of 13.07×109/L was associated with the development of Major leakage providing a sensitivity of 56% and specificity of 53%, and a diagnostic accuracy of AUC 0.635 (P=0.019) The probability of Major leakage in 13.07×109/L is 20%. At POD 5–6, similar results were found. On POD 7–8, a cutoff value of 10.37×109/L was associated with development of Major leakage, providing a sensitivity of 70% and specificity of 66%, and a high diagnostic accuracy: AUC 0.768 (P<0.001). The probability of Major leakage in this value is 18% (Table 5, Fig. 2).
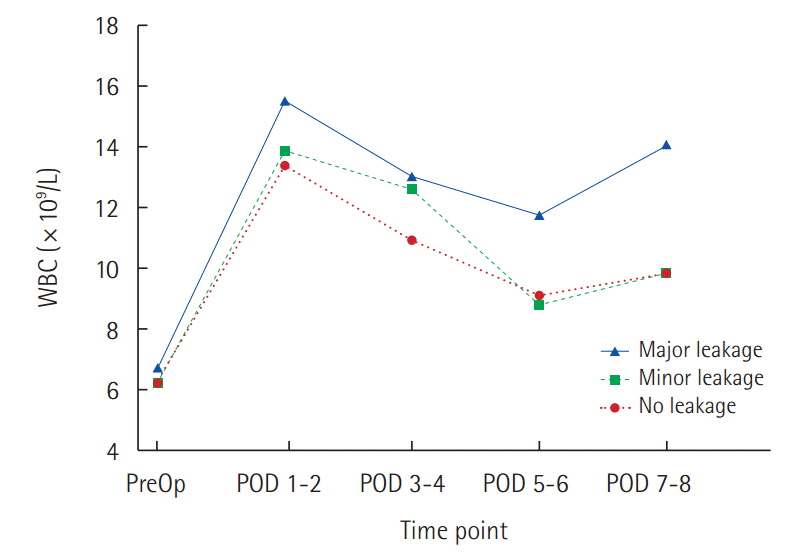
Profiles of WBC preoperatively and during POD 1–8: No leakage (dotted line) and Minor leakage (dash line) shows peaked on POD 1, and gradually decreased. Major leakage (black line) persisted during the early postoperative period. WBC, white blood cells; PreOp, preoperative; POD, postoperative day.

Relationship between serial postoperative values of WBC and development of No leakage, Minor leakage, and Major leakage
Relationship between serial postoperative values of WBC and development of No leakage, Minor leakage, and Major leakage

Receiver operating characteristics curve analysis of WBC during the postoperative course of patients after PD
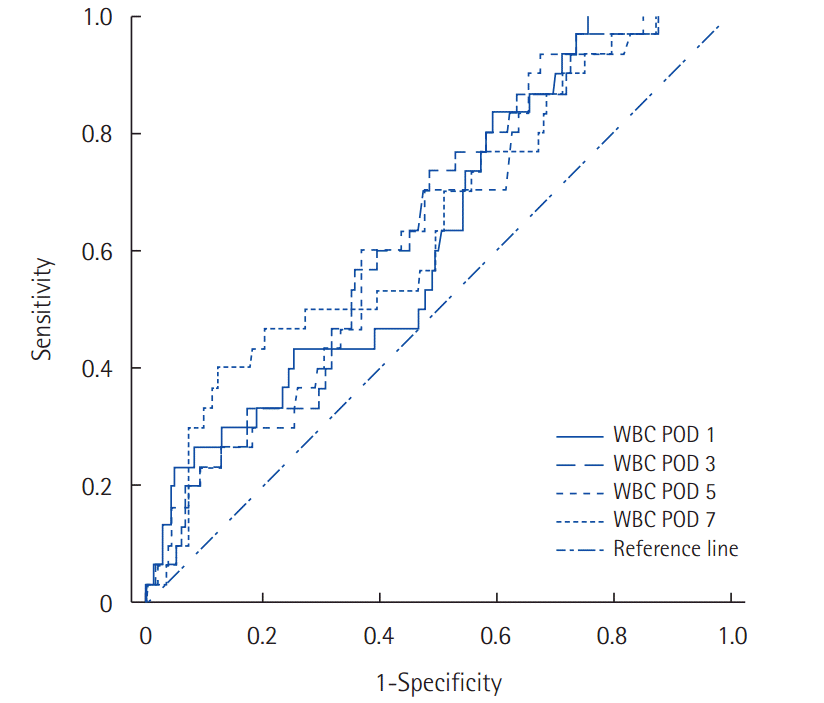
Receiver operator characteristic (ROC) curve for cutoff analysis of serum WBC from POD 1–7 in patients with POPF (B-C). The area under the curve is 0.65 on POD 1–2 (solid line), 0.65 on POD 3–4 (dashed line), 0.73 on POD 5–6 (broken line), and 0.77 on POD 7-8 (dotted line). WBC, white blood cells; POD, postoperative day; POPF, postoperative pancreatic fistula.
DISCISSION
POPF is the most important complication specific to PD (or PPPD), but it is frequently diagnosed late, often after patients have been discharged from the hospital. According to the literature, the first clinical signs of POPF are usually non-specific [17-19]. When abdominal signs become apparent, the patient is usually critically ill. This is associated with higher morbidity and mortality [20,21]. Diagnosis of POPF which requiring a re-operation is associated with high mortality rate up to 23%–67% [3,22-24]. Therefore, it is important to detect POPF before the overt signs of sepsis or peritonitis occur. This study was performed to know whether POPF can be detected early by measuring postoperative WBC counts frequently. Although WBC count was not specific factor related to morbidity, it is identified as an early predictor of septic complications after esophageal, pancreatic, and colorectal resections [25]. The observation of early persistence of increases in WBC in complicated cases demonstrates that inflammatory processes precede clinical manifestation. Recently, NLR is paid attention as an upgraded predictor of inflammatory [15]. NLR can help enhancing the accuracy of WBC. The pathogenesis of an early inflammatory response and stimulation of WBC production may be caused by local pancreatitis, tissue ischemia, or necrosis at the anastomosis in the case of POPF or by ischemia and bacterial infection of operative sites. Ischemia or intravascular release of lipopolysaccharide induces release of interleukin-6 and other cytokines, which is the stimulator for humoral and cellular components of the innate immune system. This pathway launches to eliminate damaged cells faster [26,27].
The high WBC count peak in complicated patients on POD 1–2, followed by persistence of high WBC might represent early acute phase response mechanisms, caused by a pathogenic stimulus. It is unclear if a fulminant acute phase response in the early postoperative period induces tissue regeneration or represents a harmful reaction promoting local inflammatory infiltration, tissue damage or anastomotic leak. But, Leukocytosis would tell necrotic change of the pancreaticojejunostomy at least.
There are several studies which show WBC count is the predictive factor of complications. However, the study on the relationship of WBC count, NLR and POPF is still few [15]. Previous studies have reported that WBC count on postoperative 5-7 days is associated with POPF [13-15]. Our report is uniqe that the early postoperative WBC and NLR on POD 1 or 2 is also associated POPF. The weakness of this study is to focus on WBC, NLR and POPF.
There are a few studies on the risk factors that are associated with POPF including age, gender, preoperative jaundice, operative time, intraoperative blood loss, type of pancreatic reconstruction, anastomotic technique, consistency of pancreatic stump, pancreatic duct diameter [28]. It is still unknown which of the many clinical factors are contributory to the risk of POPF development. However, our group has shown that thick and soft pancreas are risk factors [29]. Therefore, we should be cautious when performing operations with soft and thick pancreas, and it may be reasonable to measure postoperative WBC count at a regular basis.
It is a fact that high WBC count on the postoperative period is an expected and common response. However, in this study, a high WBC count and NLR at postoperative day 1-2 is a good indicator and predictor of POPF as is POD 3, 5, and 7. Although we did not mention about other factors such as C-reactive protein, albumin. There was no statistically significant difference on them.
This study is the present WBC count analysis of patients after PD and PPPD. The routine WBC count checkup is useful to detect POPF. It is important to focus on WBC count from POD 1, which can be great help to distinguish the high-risk patients and to improve the prognosis after surgery. After PD and PPPD, patients with increases of WBC count are likely to develop grade B-C. So, intensive search for complications and medical interventions may be necessary like CT scan, medications or drainage.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by Seoul National University Bundang Hospital.

